Compositions and methods for making antibodies based on use of expression-enhancing loci
A technology of gene loci and marker genes, applied in the field of improving eukaryotic cells, site-specific integration and expression, and expression of antigen-binding proteins, can solve problems such as instability and changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194] Example 1: Expression of monospecific antibodies (Abs) in two specific expression enhancing loci (via site-specific integration)
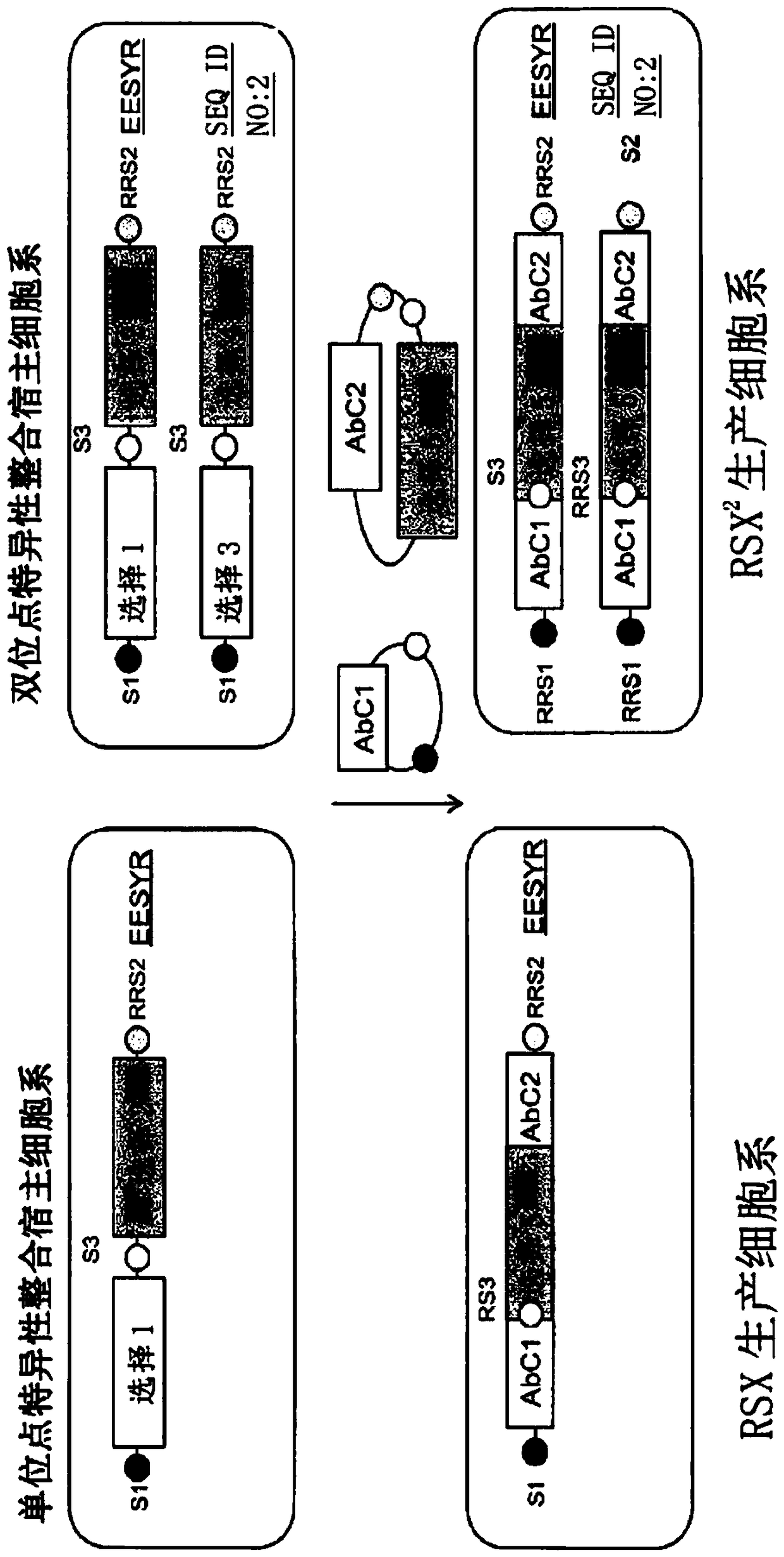
[0195] Cloning the Ab chains (AbC1, AbC2) into vectors where the RSS sites are flanked by Ab expression cassettes and expression cassettes for selectable markers, e.g. figure 1 depicted. Both Ab chains can be cloned into separate vectors or combined into one vector with 2 expression cassettes arranged in tandem in any possible order: AbC1, AbC2 and selectable markers, e.g. AbC1 is equivalent to conventional LC and AbC2 is equivalent to than conventional heavy chains.
[0196] Briefly, DNA encoding VH and VL domains can be isolated directly by PCR from single antigen-positive B cells. The heavy and light chain PCR products were cloned into Sap I linearized antibody vectors containing IgG heavy chain constant region and kappa light chain constant region, respectively. The heavy chain plasmid (AbC2) has RRS3 and RRS2 sites flanking the heavy...
Embodiment 2
[0197] Example 2: Expression of bispecific antibodies (BsAbs) in two specific expression enhancing loci (via site-specific integration)
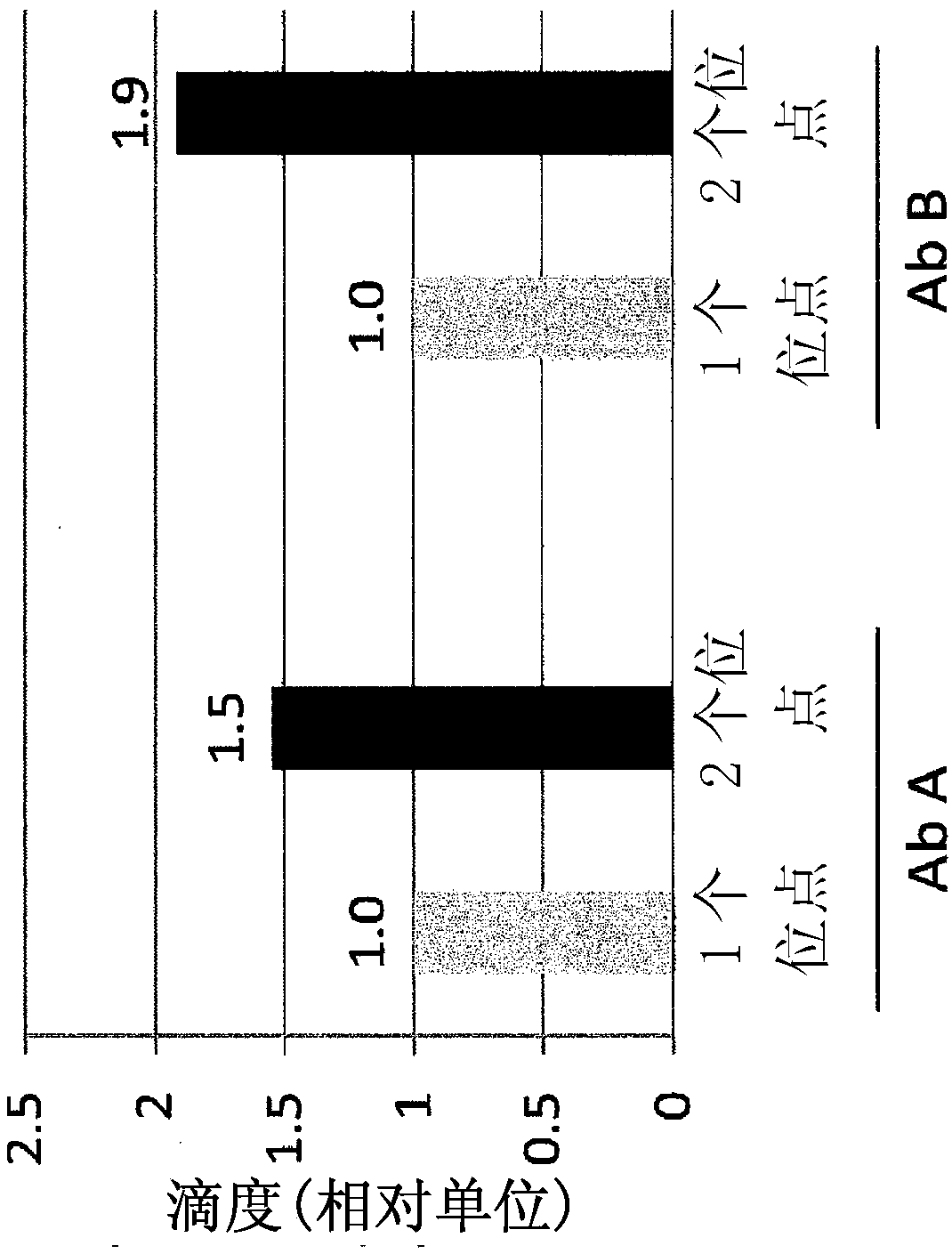
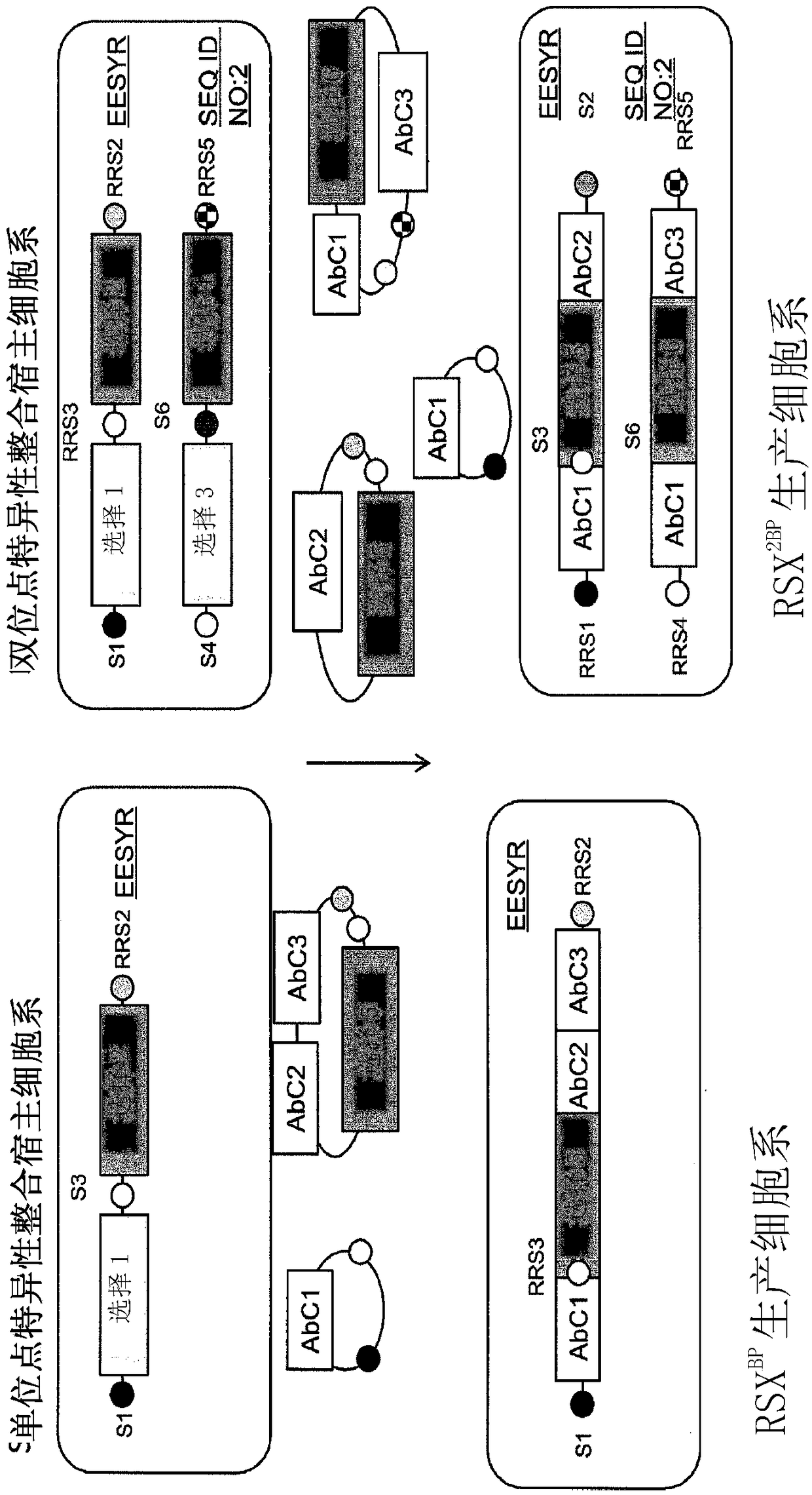
[0198] For bispecific antibody expression, the three antibody chains and two selectable markers were cloned into a plasmid similar to Example 1 such that AbC1, AbC2, and selectable marker 1 flank the first locus Compatible RRS sites, or integration sites ( SEQ ID NO:1; locus 1), and AbC1, AbC3, selectable marker 2 are compatible with the second locus or integration site (SEQ ID NO:2). In our observation, AbC1 as a conventional LC does not require two gene copies for full expression. For each site, 1 or 2 plasmids were prepared, wherein the 3 expression cassettes were arranged in tandem or into 2 plasmids, wherein the 2 expression cassettes were cloned into one vector and the remaining expression cassettes were Cloned into the second vector. see image 3 .
[0199] When recombinant plasmids expressing heavy and light chain genes are co-...
Embodiment 3
[0200] Example 3: Large scale production of bispecific and monospecific antibodies following specific integration
[0201] Host cells (CHO-K1) were generated as described above analogously to Example 1 (see also image 3 bispecific antibodies and figure 1 monospecific antibodies). will be able to transfer the gene cassette RMCE to The host cell in the locus (locus 1) and SEQ ID NO:2 (locus 2) is able to RMCE the gene cassette to only one integration site (locus 1 / locus 2). ) host cells for comparison. will carry antibody light and heavy chains (AbC1, AbC2, AbC3) and the requisite RRS and selectable marker nucleic acids (see image 3 ) vector recombined into the production cell line (RSX 2BP ) to generate host cells expressing Ab E, Ab F, Ab G, and Ab H. Thus, each bispecific antibody host cell expresses a common light chain, and two heavy chains that bind different antigens, wherein the one heavy chain is engineered in its CH3 domain to differentially bind protein A (e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com