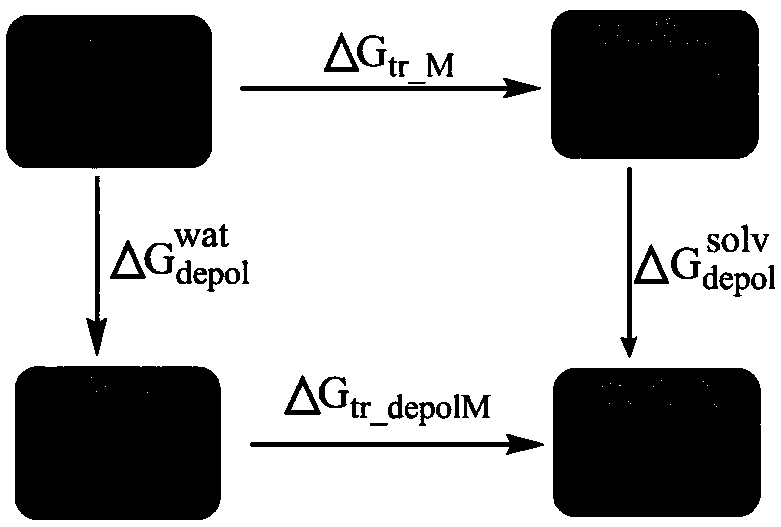
Method of calculating distributing constant of compound in water and any solvent
A technology for assigning constants and compounds, applied in the field of computational chemistry, to solve problems such as misleading, inability to accurately predict the physical and chemical properties of compounds, and inability to accurately predict the ADMET performance of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Water / n-octanol partition constant (logP oct ) calculation:
[0070] We use 242 with logP oct Compounds with values are used to build the model to obtain the constant k 1 , k 2 , k 3 and c 1 , with 245 logP oct Compounds with values were used to verify the accuracy of the model.
[0071] with 242 logP oct The value of the compound's ΔG alk tr_depol , H HBD , H HBA and logP oct The model established by the method of multiple linear regression for the data is:
[0072] logP oct =0.1482*ΔG alk tr_depol +0.00385*H HBD –0.1188*H HBA +0.1159 (1)
[0073] N=242,R 2 = 0.985, stdev = 0.234.
[0074] Formula (1) was used to calculate the logP of 245 compounds oct value, its calculated value [logP oct (calc)] and experimental value [logP oct (obsv)] The relationship is as Figure 5 shown. Depend on Figure 5 It can be seen that the calculated value is very close to the actual value, indicating that the method of the present invention can be used to ac...
Embodiment 2
[0076] Water / chloroform partition constant (logP chl ) calculation:
[0077] with a logP of 80 chl Compounds of value are modeled using 75 with logP chl Compound validation model for values.
[0078] with a logP of 80 chl The value of the compound's ΔG alk tr_depol , H HBD , H HBA and logP chl The model established by the method of multiple linear regression for the data is:
[0079] logP chl =0.1632*ΔG alk tr_depol –0.1625*H HBD –0.1016*H HBA +0.3220 (2)
[0080] N=80,R 2 = 0.978, stdev = 0.287.
[0081] Formula (2) was used to calculate the logP of 75 compounds chl value, its calculated value [logP chl (calc)] and experimental value [logP chl (obsv)] The relationship is as Figure 6 shown. Depend on Figure 6 It can be seen that the calculated value is very close to the actual value, indicating that the method of the present invention can be used to accurately calculate the distribution constant.
Embodiment 3
[0083] Water / n-hexadecane partition constant (logP 16 ) calculation:
[0084] with 166 logP 16 Compounds of value are modeled with 160 logP 16 Compound validation model for values.
[0085] with 166 logP 16 The value of the compound's ΔG alk tr_depol , H HBD , H HBA and logP 16 The model established by the method of multiple linear regression for the data is:
[0086] logP 16 =0.1729*ΔG alk tr_depol –0.1731*H HBD –0.1748*H HBA +0.0342 (3)
[0087] N=166,R 2 = 0.997, stdev = 0.155.
[0088] Formula (3) was used to calculate the logP of 160 compounds 16 value, its calculated value [logP 16 (calc)] and experimental value [logP 16 (obsv)] The relationship is as Figure 7 shown. Depend on Figure 7 It can be seen that the calculated value is very close to the actual value, and the parameter ΔG alk tr_depol , H HBD and H HBA The water / n-hexadecane partition constant of a compound can be accurately predicted from its structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com