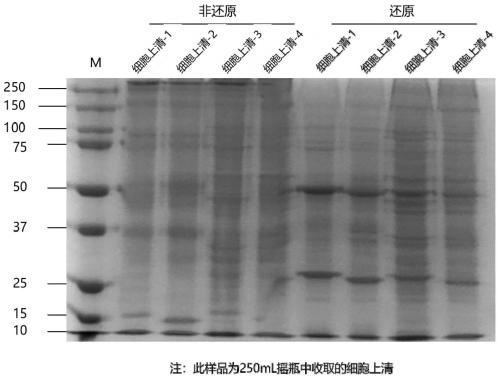
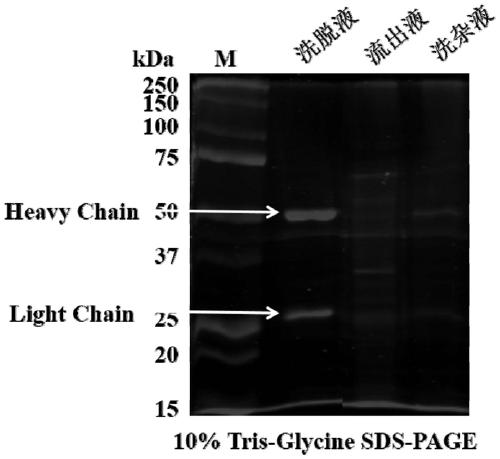
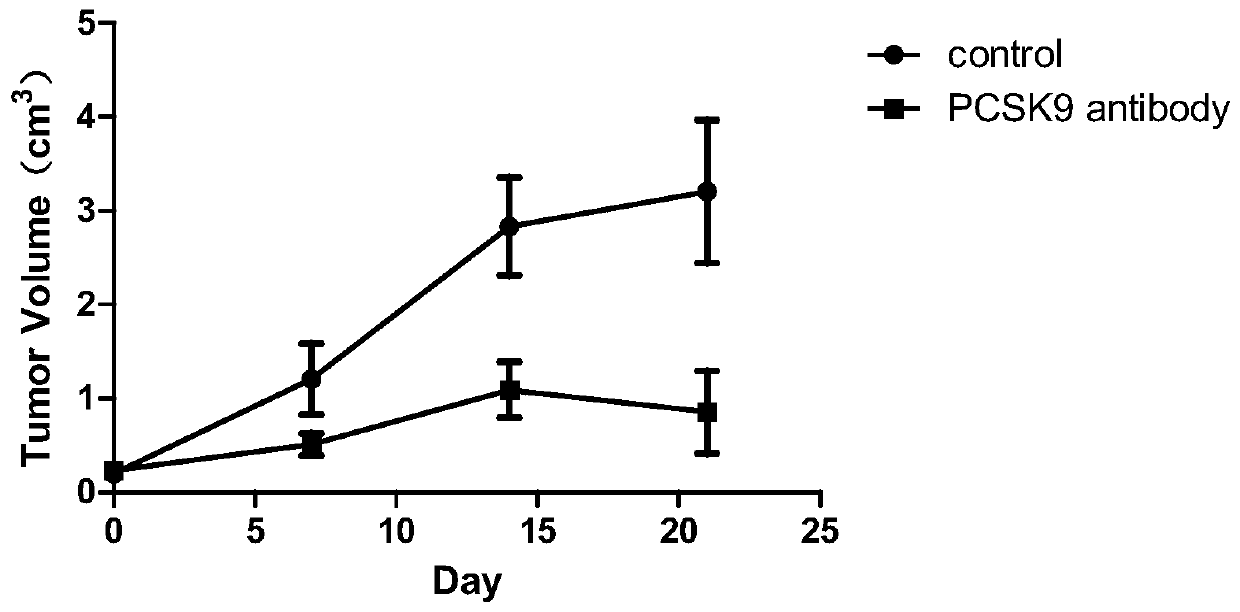
Antihuman PCSK9 monoclonal antibody and application thereof
A monoclonal antibody and fully human antibody technology, which is applied in the direction of antibodies, antibody medical components, anti-tumor drugs, etc., can solve the problems of low output, long time, low antibody purity, etc., and achieve the effect of high output and short cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Screening Fab phage library to obtain anti-human PCSK9 positive monoclonal
[0030]1. Dilute the PCSK9 protein (GenScript Biotechnology Co., Ltd.) to a concentration of 100ug / ml, add 1.5ml to the immunotube, and coat overnight at 4°C. On the next day, the coating solution in the immunotube was discarded and washed 3 times with PBS. Prepare 2% skimmed milk powder to seal the immunotube for 3-4 hours, and at the same time display the natural human Fab phage constructed by us according to the report of Hans]. W.de Haard et al. (Antibody PhageDisplay, 2002, p87-100) The library was also blocked for 1 hour, and then the phages were added to the immune tube, turned over repeatedly for 2 hours, and the phages bound to the immune tube were eluted with 0.1% Tween 20, and neutralized. Infect TG1 with the neutralized phages to prepare for the next round of screening.
[0031] 2. After 3-5 rounds of screening, the eluted phages were infected with Escherichia coli TG1 (B...
Embodiment 2
[0044] Example 2 Expression and Purification of Positive Monoclonal PA6 Antibody Fab Fragment
[0045] 1. The screened PA6 antibody with high affinity was infected with TG1 and amplified, then the bacterial liquid was collected, the plasmid was extracted using a plasmid extraction kit (omega, D6950-01), and Gene III of the plasmid was excised.
[0046] 2. Transform the obtained GeneIII excised plasmid into Escherichia coli TG 1, 2YTA medium, culture to logarithmic phase at 37°C, add 1mM IPTG, induce expression overnight at 37°C.
[0047] 3. The supernatant was collected by centrifugation, and purified using Protein A (Kangwei Century, CW0894S) to obtain PA6Fab protein.
Embodiment 3
[0048] Example 3 Expression and purification of positive clones in the form of full-length antibody IgG1
[0049] 1. Construction of the heavy chain expression plasmid: Amplify the CH1-VH part of the Fab fragment of the PA6 antibody by PCR and combine the CH1-VH part with the Fc fragment stored in the laboratory (see fully human monoclonal antibody against PCSK9 for details). The variable region gene and its application, Patent No. CN 104861071A) was ligated using T4 ligase. A Kozak sequence (AAG CTT GCCACC), signal peptide and restriction enzyme sites were then added to the N-terminus of the heavy chain variable region VH. The amplified heavy chain fragment and the expression vector UCOE-Mu-P were digested with restriction endonucleases NgoMIV and NheI, and the fragment was connected to the vector after digestion.
[0050] 2. Construction of the light chain expression plasmid: the complete light chain part of the Fab fragment of the PA6 antibody was amplified by PCR. A Koza...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap