Progesterone receptor antagonist dosage form
A dosage form and drug technology, applied in the field of progesterone receptor antagonist dosage forms, can solve problems such as being limited to 6 months
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0059] exist first embodiment In, the oral dosage form additionally comprises pharmaceutically acceptable excipients.
[0060] exist second embodiment In the present invention, the oral dosage form additionally comprises pharmaceutically acceptable excipients and / or at least one or more other active substances, especially active substances known for the treatment and / or prevention of the aforementioned diseases.
[0061] Embodiments and preferred features as described above are included therein.
[0062] In a fourth aspect, the present invention relates to an oral dosage form as described in the third aspect for use in the treatment and / or prevention of gynecological diseases. The gynecological disorder is preferably characterized by excessive uterine bleeding. More preferably, the gynecological disorder is uterine fibroids (fibroids, leiomyomas), endometriosis or excessive menstrual bleeding. Even more preferably, the gynecological disease is uterine fibroids (fibroids,...
Embodiment 1
[0084] Embodiment 1: the synthetic route of compound 1
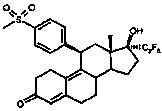
[0085] (11β,17β)-17-Hydroxy-11-[4-(methylsulfonyl)phenyl]-17-(pentafluoroethyl)estr-4,9-dien-3-one
[0086]
[0087] 5 g of the compound described in Example 1b) were dissolved in a mixture of 140 ml THF and 140 ml methanol. A solution of 20 g of Oxone® in 94 ml of water was slowly added dropwise at 0 °C. This was followed by further stirring at 0°C for 3.5 hours. A mixture of water and dichloromethane was then added to the reaction mixture. The phases were separated and the aqueous phase was extracted several times with dichloromethane. The combined organic phases were washed with saturated aqueous sodium chloride, dried over sodium sulfate and concentrated under vacuum. The crude product was purified by silica gel chromatography. 3.8 g of the title compound are obtained.
[0088] 1 H-NMR (300 MHz, CDCl 3 ): δ= 7.86 d (2H); 7.40 d (2H); 5.81 sbr (1H); 4.50 dbr (1H); 3.07 s (3H); 0.51 s (3H).
Embodiment 2
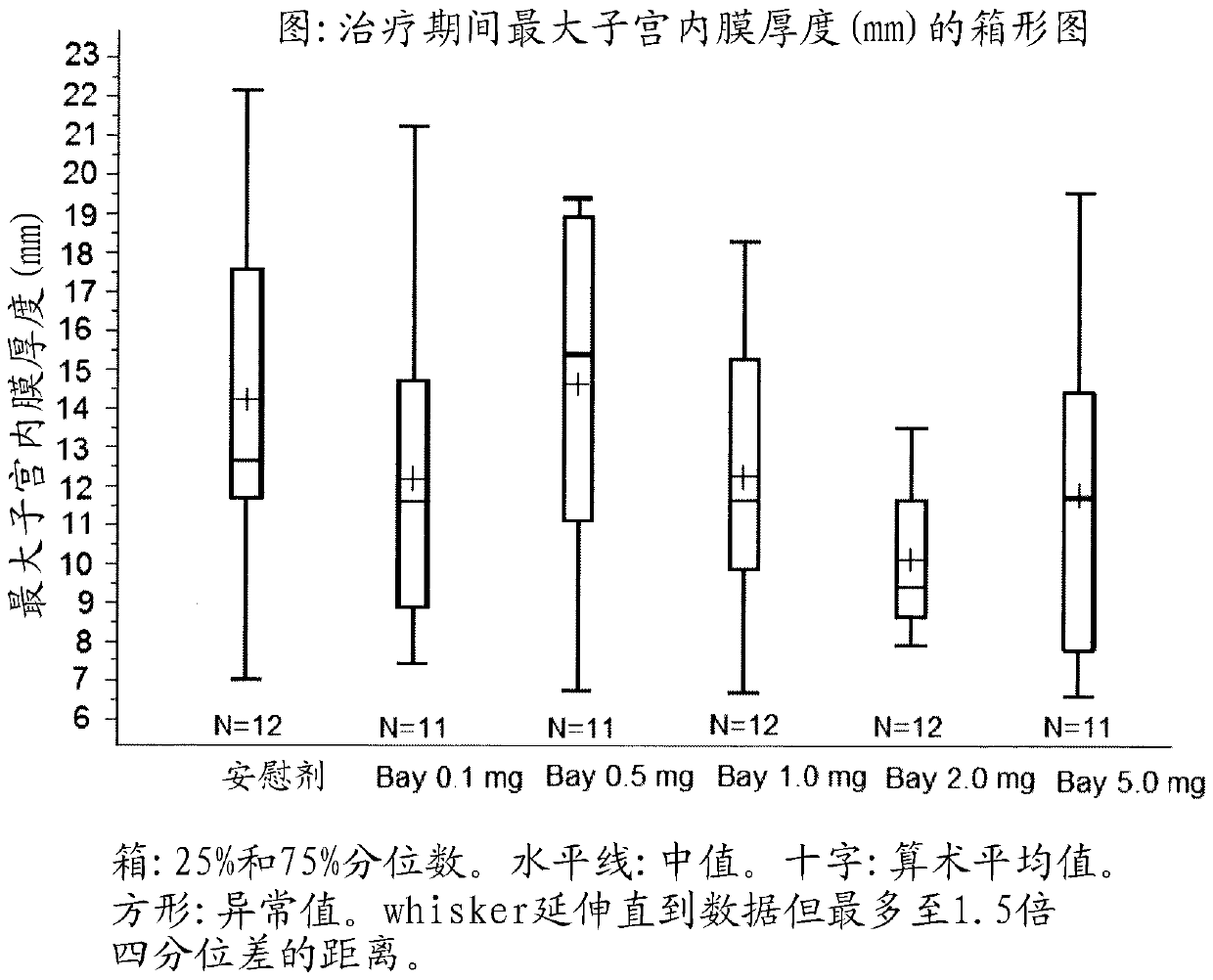
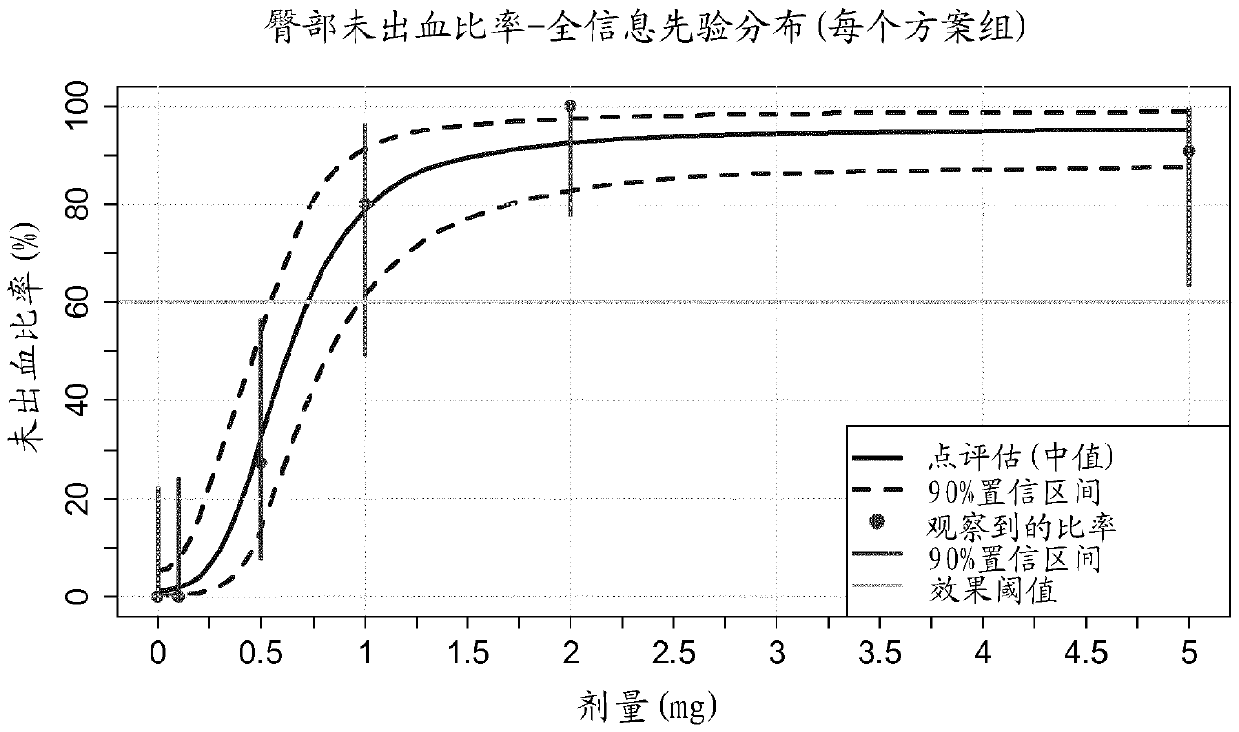
[0089] Example 2: Dose Effect of Treatment with Compound 1 for 84 Days
[0090] Random Study Population:
[0091] 1. Healthy female subjects, sterilized by tubal ligation
[0092] 2. Screening age: 18-45 years old
[0093] 3. Screened Body Mass Index (BMI): ≥ 18 and ≤ 32 kg / m²
[0094] 4. At least 3 consecutive normal menstrual periods with a cycle length of 24-35 days prior to the first screening examination based on the subject's history
[0095] 5. Absence of Clinically Relevant Abnormal Findings in Pretreatment Endometrial Biopsy
[0096] 6. Adequate venous access (frequent blood draws).
[0097] Program:
[0098] Table 1: Therapeutic Doses Using Compound 1
[0099] .
[0100] After the pre-treatment cycle, subjects must start taking the study drug on the first or second day of menstrual bleeding. A pretreatment cycle commencing on the first day of a subject's menstrual bleeding following screening checks has indicated that the subject is eligible for furth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com