In vivo drug delivery devices and methods for drug delivery
A technology for delivering devices and medicines, applied in the directions of medicine delivery, medicine devices, medicine formulations, etc., can solve the problems of lack of desired biocompatibility, stability, sterilization, manufacturability, wall thickness, flexibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
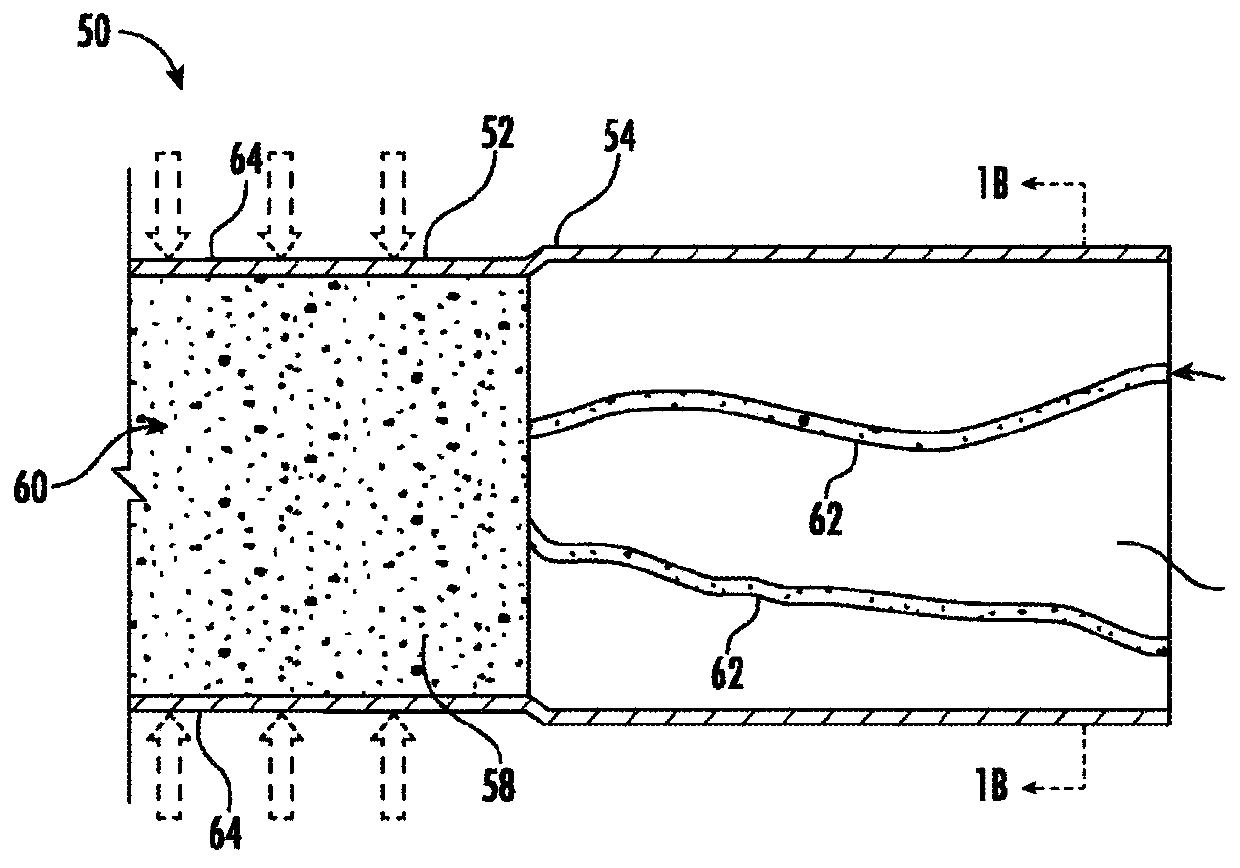
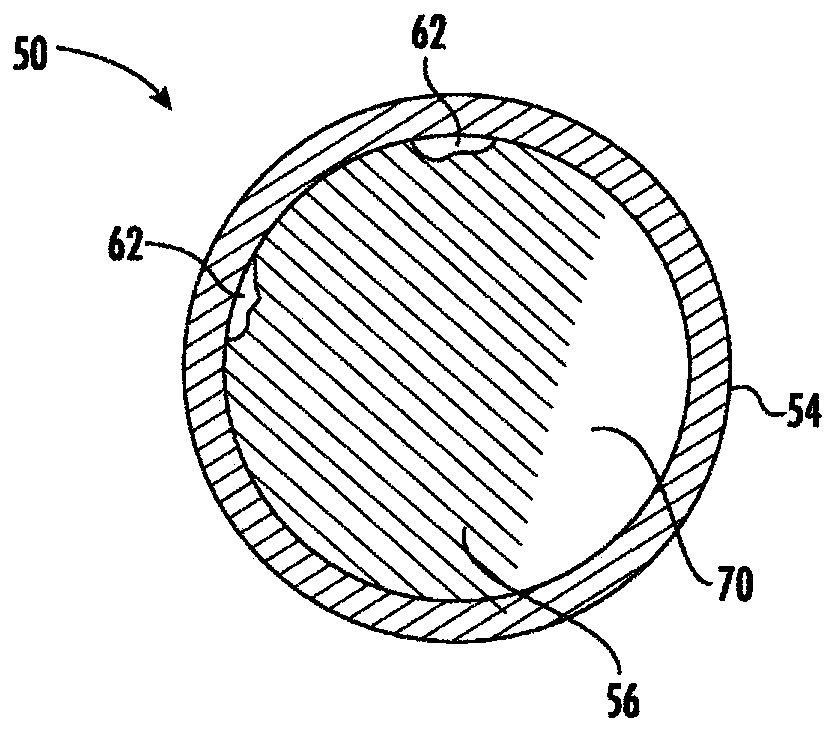
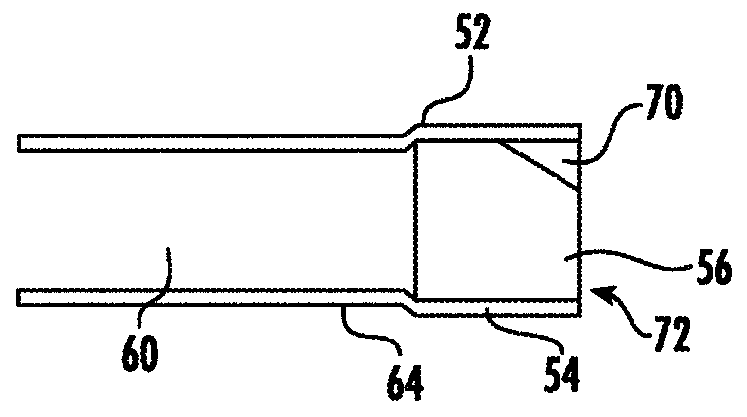
[0158] A device prototype with a central laser-drilled orifice was fabricated and loaded with trospium chloride tablets. One set of devices contained two gasket apertures at each end (ie, a plug with a longitudinal aperture formed therein), while a second set of devices had two restrictive plugs at the ends. exist Figure 11 and 12 A schematic diagram of the prototype is shown in . Figure 11 The device in has three drug release openings: two at opposite ends and one on the side wall. The drug reservoir lumen of the device has an inner diameter of 2.64 mm and a wall thickness of 0.2 mm. The wall has a hardness of 50A. Figure 12 The device in has a release opening, plus two opposing ends capable of providing release when the hydrostatic pressure is sufficient to form a microchannel. The drug reservoir lumen of the device has an inner diameter of 2.64 mm and a wall thickness of 0.2 mm. The wall has a hardness of 50A.
[0159] The device was placed in a container of deion...
example 2
[0161] The device shown in Fig. 3 was fabricated in the following manner. The device is a dual-lumen silicone tube with a laser-machined orifice, a polymeric bismuth-coated tube to contain the drug and form a one-way valve at each end of the drug compartment in the drug lumen. A silicone elastomer plug of toluene C, and a white silicone adhesive used to hold the plug in place in the large lumen, a preformed superelastic nitinol circular section resistant to the lumen of the retaining frame filament, and retaining lumen ends sealed with a translucent silicone adhesive. The drug is formulated as a drug containing trospium chloride (active pharmaceutical ingredient), povidone (polyvinylpyrrolidone (PVP)) K29 / 32 (adhesive excipient) and polyethylene glycol 8000 (lubricant excipient) tablet. Each device contains 850 mg of trospium chloride. The device is small in size (less than 5 cm in the long axis), flexible and contoured in order to minimize potential allergies and inflammat...
example 3
[0165] Following the parameters in Table 1, a trospium chloride releasing intravesical device was fabricated for in vitro release characterization. The device was fabricated using one of two types of silicone housing components. One type (RW) comprises an annular tube with a wall thickness of 0.2 mm and a durometer of 5OA defining a drug reservoir lumen. Another type (TW) comprises an annular tube with a wall thickness of 0.4mm and a durometer of 35A defining a drug reservoir lumen. Both types of silicone parts have a drug reservoir lumen inner diameter of 2.64 mm. All devices had a 150 μm diameter laser drilled orifice approximately centered on the sidewall of the silicone part. The system is assembled with plugs of different lengths. The system either contains two plugs, one at each end, or one plug with a gasket on the other end of the system to seal that end of the reservoir (i.e., so that microchannels cannot form on that end. ). The drug reservoir lumen of each syst...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap