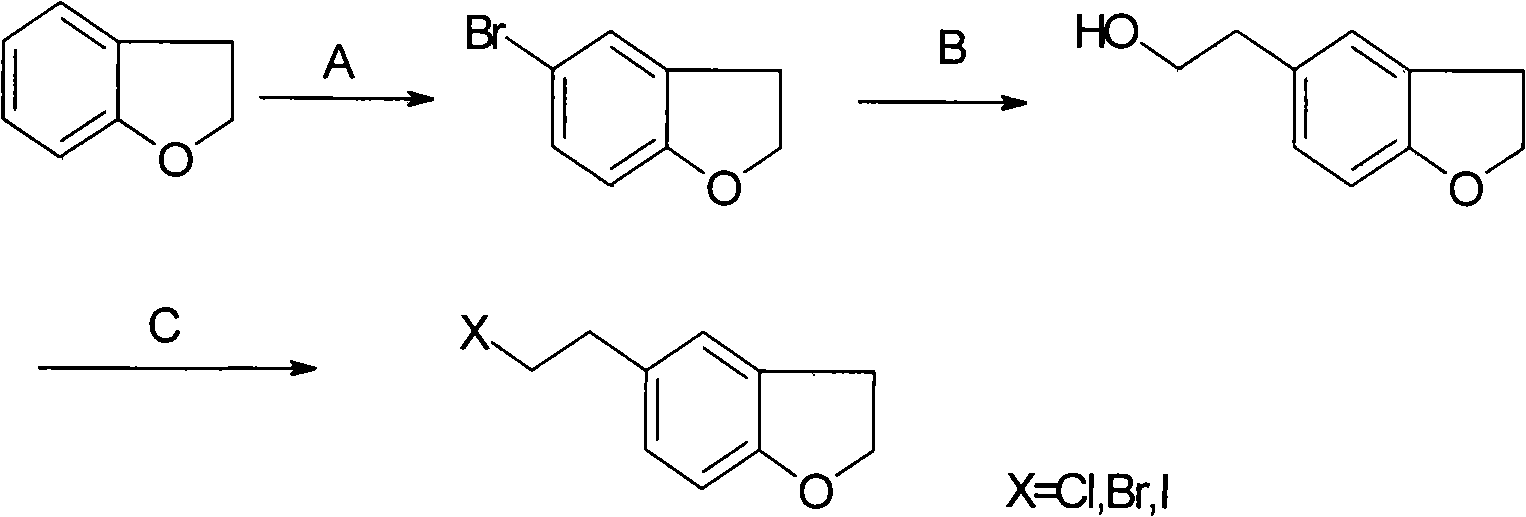
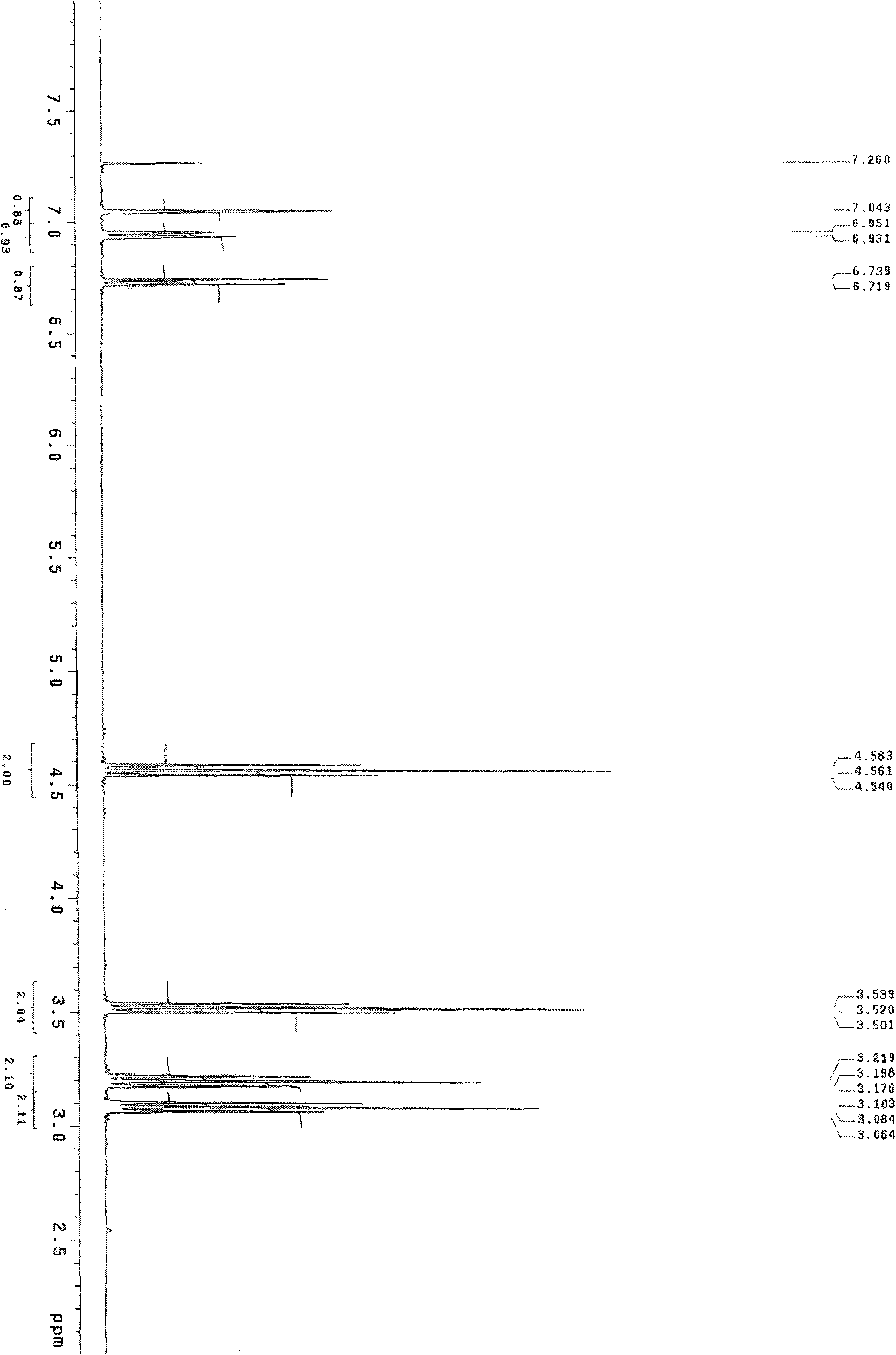Preparation of darifenacin intermediate 5-(halogenated ethyl)-2,3-dihydrobenzofuran
A technology of haloethyl and dihydrobenzene, which is applied in the field of chemical synthesis, can solve the problems of needing dangerous reagents, difficulty in industrialization, and high cost, and achieve the effect of novel process route, low production cost, and no three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Synthesis of 5-bromo-2,3-dihydrobenzofuran
[0030] Dissolve 6 grams of 2,3-dihydrobenzofuran in 250 milliliters of dichloromethane, at room temperature, add dropwise a solution of 48 grams of liquid bromine in 50 milliliters of dichloromethane within 45 minutes, keep stirring for 30 minutes after adding, The reaction was quenched by adding 50 ml of sodium bisulfite aqueous solution, and the layers were separated. The organic layer was washed with 100 ml of water, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 5-bromo-2,3-dihydrobenzofuran as a yellow solid. The melting point is 51-52°C.
[0031] (2) Synthesis of 2,3-dihydrobenzofuran-5-ethanol
[0032] 1.7 g (70 mmol) of magnesium chips and 20 ml of tetrahydrofuran were heated to reflux, 5 drops of 1,2-dibromoethane were added, and 12.4 g (62.3 mmol) of 5-bromo-2,3-dihydro 40 ml of tetrahydrofuran solution of benzofuran, keep refluxing until the magnesium chips basically disappear. Co...
Embodiment 2
[0043] Step (1), step (3) are the same as embodiment 1, the difference is:
[0044] Step (2): Synthesis of 2,3-dihydrobenzofuran-5-ethanol
[0045]1.7 g (70 mmol) of magnesium chips and 30 ml of anhydrous ether were heated to reflux, 5 drops of 1,2-dibromoethane were added, and 12.4 g (62.3 mmol) of 5-bromo-2,3- 40 milliliters of tetrahydrofuran solution of dihydrobenzofuran, then keep reflux until the magnesium chips disappear substantially. Cool, keep the temperature not exceeding 0°C, feed ethylene oxide, keep feeding for 30 minutes, then heat to reflux for 6 hours, cool to 25°C, inject the reaction solution into 75 ml of saturated ammonium chloride aqueous solution. The organic layer was separated, and the aqueous layer was extracted twice with a small amount of ether. The organic layers were combined, dried, filtered, and the filtrate was concentrated under reduced pressure to obtain 10.2 g of yellow oily 2,3-dihydrobenzofuran-5-ethanol.
Embodiment 3
[0047] Step (1), step (2) are the same as embodiment 1, the difference is:
[0048] Step (3): 5-(Chloroethyl)-2,3-dihydrobenzofuran
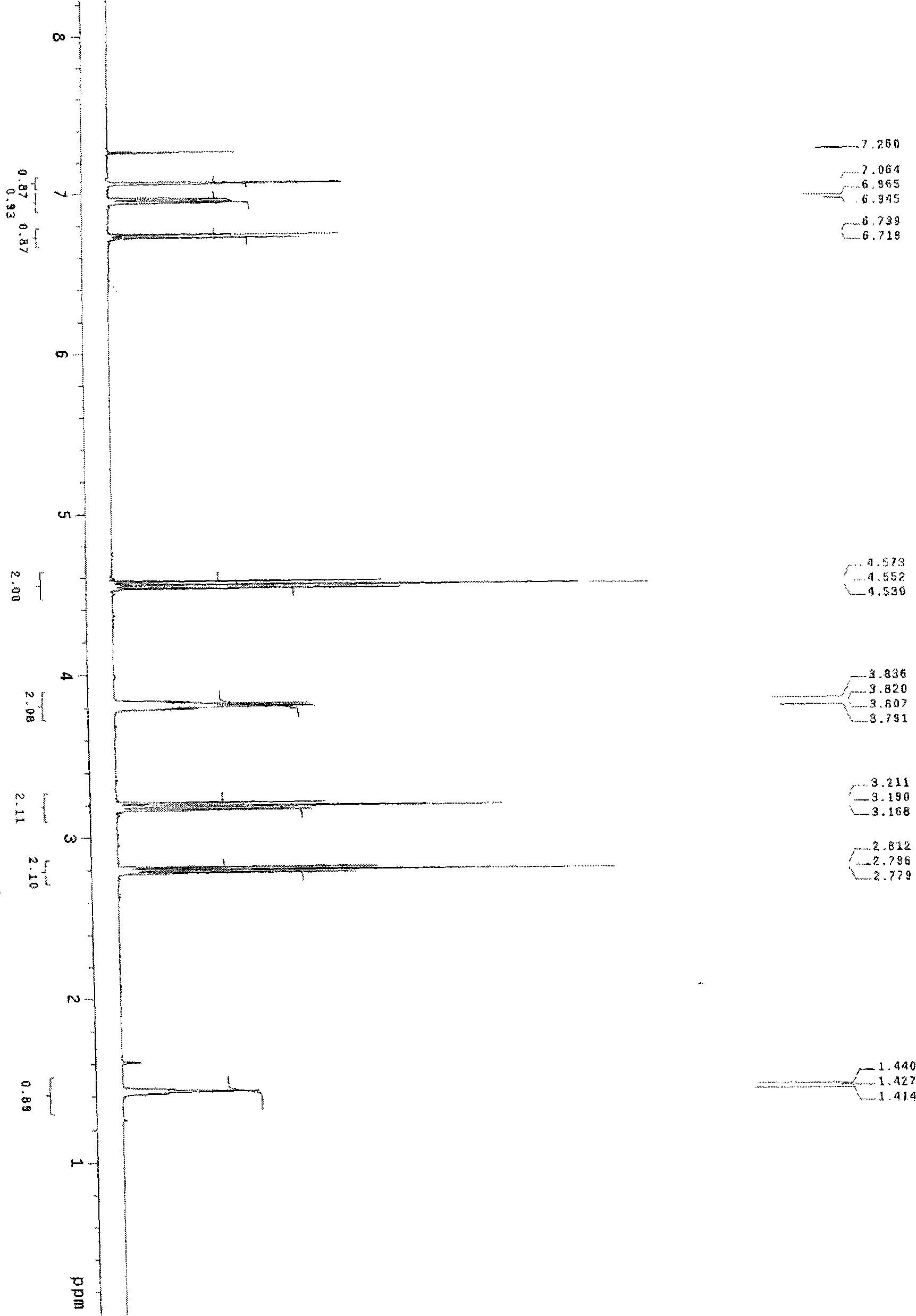
[0049] Add 0.612 g of 2,3-dihydrobenzofuran-5-ethanol dropwise to 10 ml of thionyl chloride solution, heat to reflux for 3 hours, recover excess thionyl chloride, cool to room temperature, add 20 ml of dichloromethane Methane, 20 milliliters of 10% sodium carbonate solution was added dropwise, the layers were separated, the aqueous layer was extracted with 20 milliliters × 2 of dichloromethane, the organic layers were combined, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain an oil, which was solidified on standing. Obtained 0.32 g of 5-(chloroethyl)-2,3-dihydrobenzofuran with a melting point of 49-50° C. (see figure 2 ).
[0050] The corresponding data of the 1HNMR spectrum of 5-(2-bromoethyl)-2,3-dihydrobenzofuran in this example are as follows: 1HNMR (CDCl3), δ=7.04 (s, 1H); 6.95-6.93 (d, 1H); 6.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap