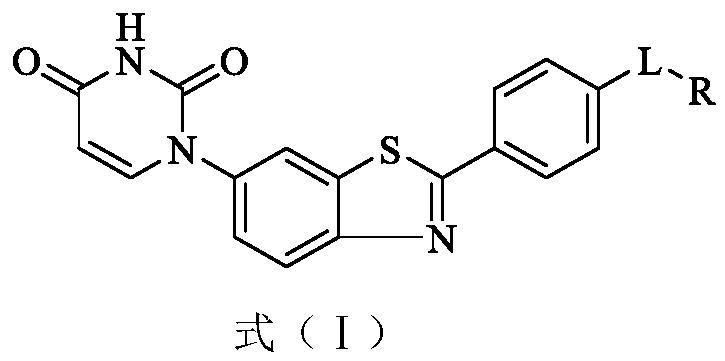
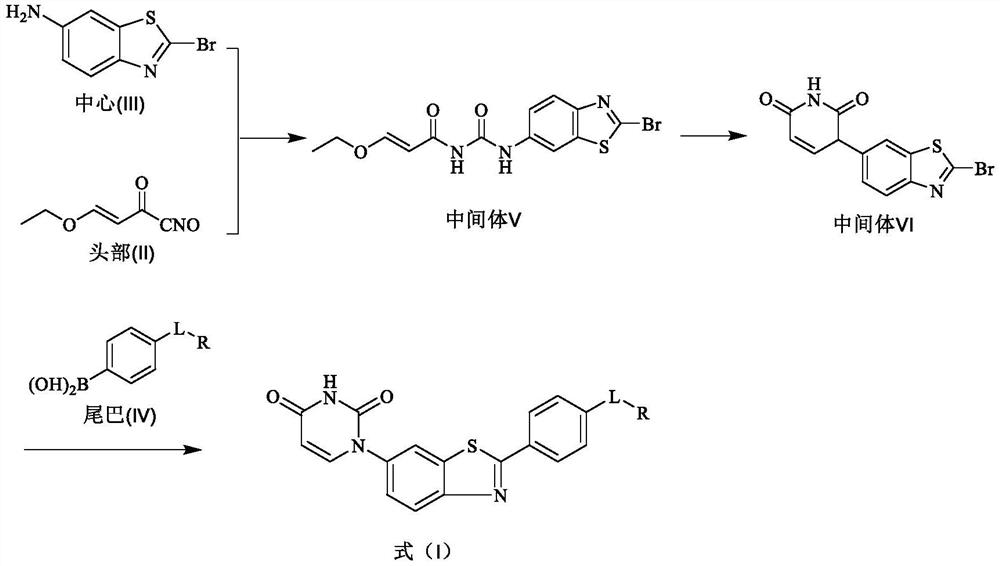
One class with urine-pyrine-pyrazole structure derivatives, their preparation methods and anti-HCV drugs
A technology for benzothiazole and derivatives is applied in the field of preparing anti-hepatitis C drugs, and achieves the effects of reasonable route design, good inhibitory activity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
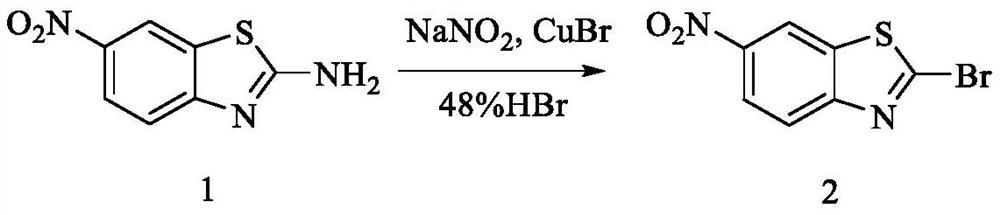
[0042] Example 1 Synthesis of 2-bromo-6-nitro-benzo[d]thiazole (compound 2)
[0043]
[0044] 2-Amino-6-nitrobenzothiazole (10.0 g, 51.0 mmol) and CuBr (0.88 g, 6.1 mmol) were suspended in 48% aqueous HBr (100 mL) and water (90 mL), and NaNO was added slowly 2 (30.5g, 442mmol), continue to react at room temperature for 1 hour, extract with DCM and EtOAC respectively after the reaction to obtain the crude product, then recrystallize with isopropanol, filter and dry. Yield 88.4%; 1 H-NMR (400MHz, CDCl 3): δ = 8.78 (d, J = 2.0Hz, 1H), 8.38 (dd, J = 1.6Hz, 7.2Hz, 1H), 8.11 (d, J = 7.2Hz, 1H).
Embodiment 2
[0045] Example 2 Synthesis of 2-bromo-6-amino-benzo[d]thiazole (compound 3)
[0046]
[0047] Compound 2 (45mmol, 11.6g), Fe (225mmol, 12.6g), NH 4 Cl (135mmol, 7.22g) was placed in a 500mL round-bottomed flask, added to absolute ethanol (150mL) and water (50mL), under nitrogen protection, heated to reflux at 100°C for 2-3h, and filtered with diatomaceous earth after the reaction was completed , filtrate with K 2 CO 3 After adjusting the base, extract with DCM (50 mL×2), evaporate the solvent under reduced pressure, and recrystallize from isopropanol to obtain compound 3. Yield 78.2%; 1 H-NMR (400MHz, d 6 -DMSO): δ=7.61(d, J=7.6Hz, 1H, Ar), 7.05(d, J=2.0Hz, 1H, Ar), 6.76(dd, J=1.6Hz, 6.8Hz, 1H, Ar) ,5.53(s,2H,NH).
Embodiment 3
[0048] Example 3 Synthesis of (E)-N-((2-bromobenzo[d]thiazol-6-yl)carbamoyl)-3-ethoxyacrylamide (compound 6)
[0049]
[0050] Silver isocyanate (12.0g, 80mmol) at 100°C with P 2 o 5 Vacuum-dry, add anhydrous toluene 80mL, nitrogen protection, avoid light, heat to reflux for 0.5h; 3-ethoxyacryloyl chloride (4.82g, 40mmol) is dissolved in anhydrous toluene 15mL, and slowly dropwise added to the above reaction solution , after continuing to heat and reflux for 0.5h, react at room temperature for 3h;
[0051] Compound 3 (1.64g, 7.14mmol) was dissolved in anhydrous DMF solution (60mL), and the supernatant of the above reaction solution was slowly added to the reaction solution, and reacted overnight at room temperature. Chromatographic separation (EtOAC: PE5: 3) gave compound 6 with a yield of 71.9%; 1 H-NMR (400MHz, CDCl 3 ):δ=11.1(s,1H,Ar),10.6(s,1H,Ar), 8.43(d,J=2.0Hz,1H,CH),7.93(d,J=7.2Hz,1H,CH), 7.71(dd, J=2.0Hz, 7.2Hz, 1H, Ar), 7.55(q, J=2.0Hz, 7.2Hz, 1H, NH), 5.61(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com