Detection of misfolded tau protein
A misfolding, protein technology, applied in the field of detection of misfolded TAU protein, can solve problems such as impracticality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1: Preparation of synthetic Aβ oligomers
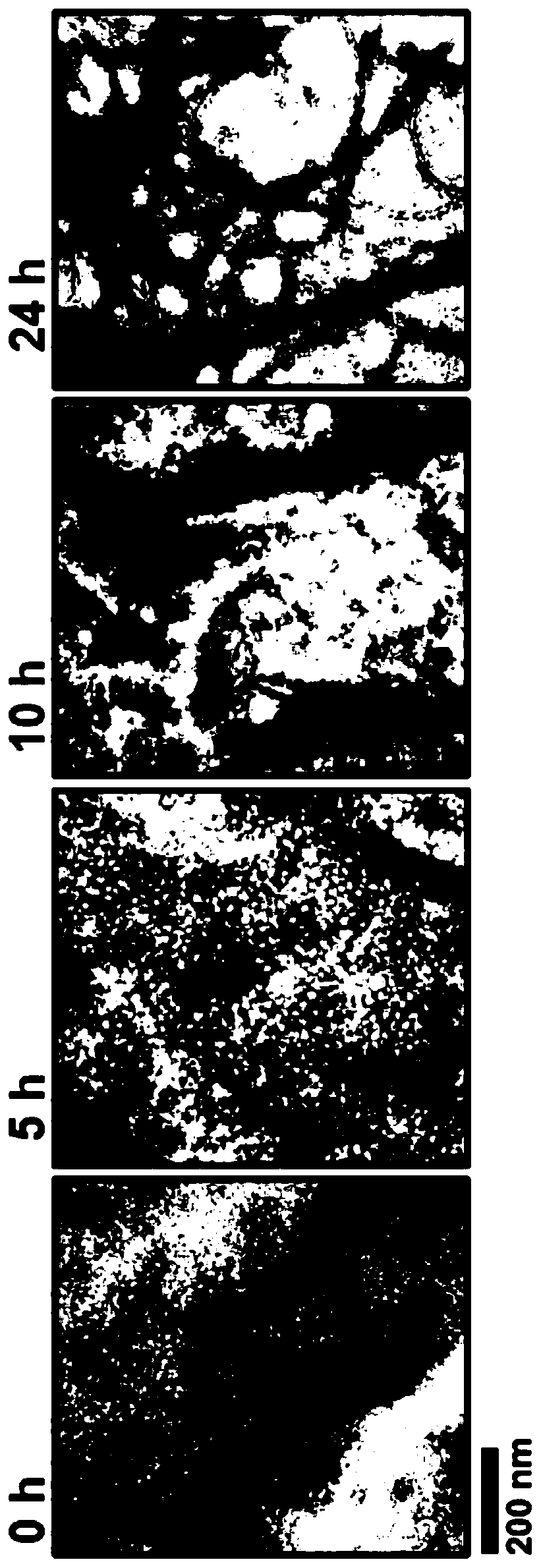
[0150] Aβ1-42 was synthesized using solid-phase N-tert-butoxycarbonyl chemistry at the W. Keck facility at Yale University and purified by reverse-phase HPLC. The final product was lyophilized and characterized by amino acid analysis and mass spectrometry. To prepare stock solutions free of aggregated, misfolded Aβ protein, aggregates were dissolved at high pH and filtered through a 30 kDa cut-off filter to remove residual aggregates. To prepare different types of aggregates, seedless Aβ1-42 (10 μM) solutions were incubated at 25° C., 0.1 M Tris-HCL, pH 7.4 for different times under agitation. The preparations included A[beta] monomers and mixtures of fibrils, protofibrils and soluble misfolded A[beta] protein in different ratios depending on the incubation time. The degree of aggregation was characterized by ThT fluorescence emission, electron microscopy after negative staining, dot blot studies with A11 conformation...
Embodiment 2
[0153] Example 2: Detection of synthetic Aβ oligomers by Aβ-PMCA
[0154] Example 2A. Seeding of Aβ aggregation by using or not using different amounts of synthetic soluble misfolded Aβ protein (control (no Aβ oligomers); or 3 of synthetic soluble misfolded Aβ protein in the presence of Thioflavin T 80, 300, and 8400 femtomoles) were incubated with seed-free Aβ1-42 solutions for study. Aβ-PMCA general procedure: 2 μM aggregate-free Aβ1-42 solution in 0.1M Tris-HCL pH7.4 (total volume 200 μL) was placed in an opaque 96-well plate and incubated alone or after synthetic Aβ aggregates ( Prepared by incubating for 5 h as described in Example 1) or in the presence of a 40 μL aliquot of CSF. Samples were incubated in the presence of 5 μM Thioflavin T (ThT) at a constant temperature of 22°C with cyclic agitation using an Eppendorf thermomixer (1 min at 500 RPM followed by 29 min without shaking). At various time points, ThT fluorescence was measured in 485 nm plates after excitation...
Embodiment 2B
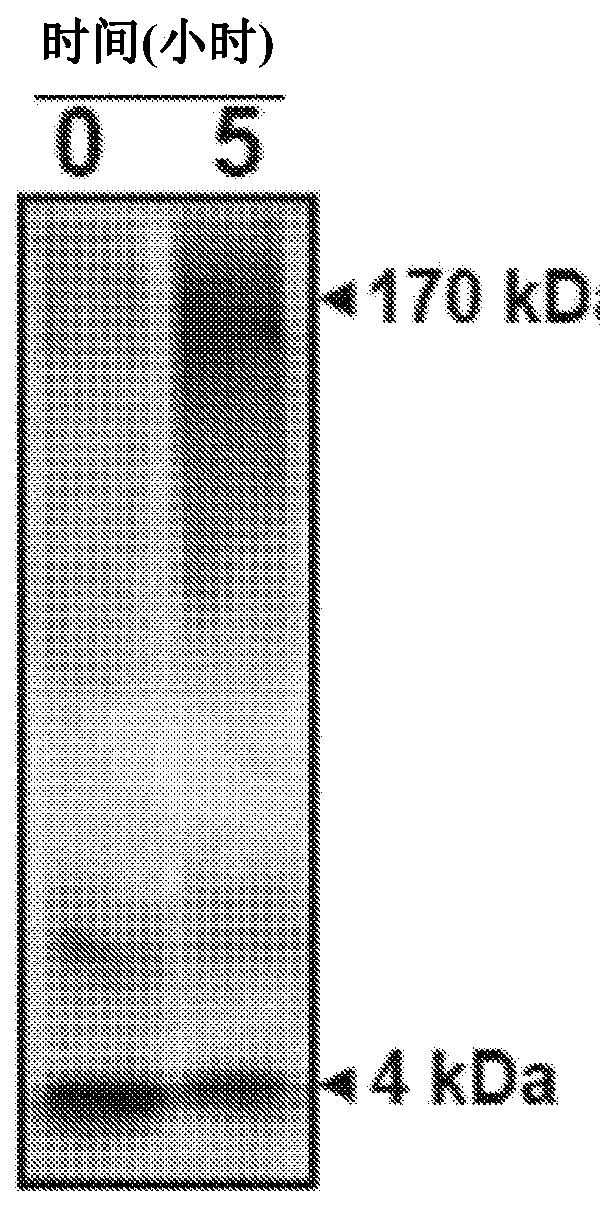
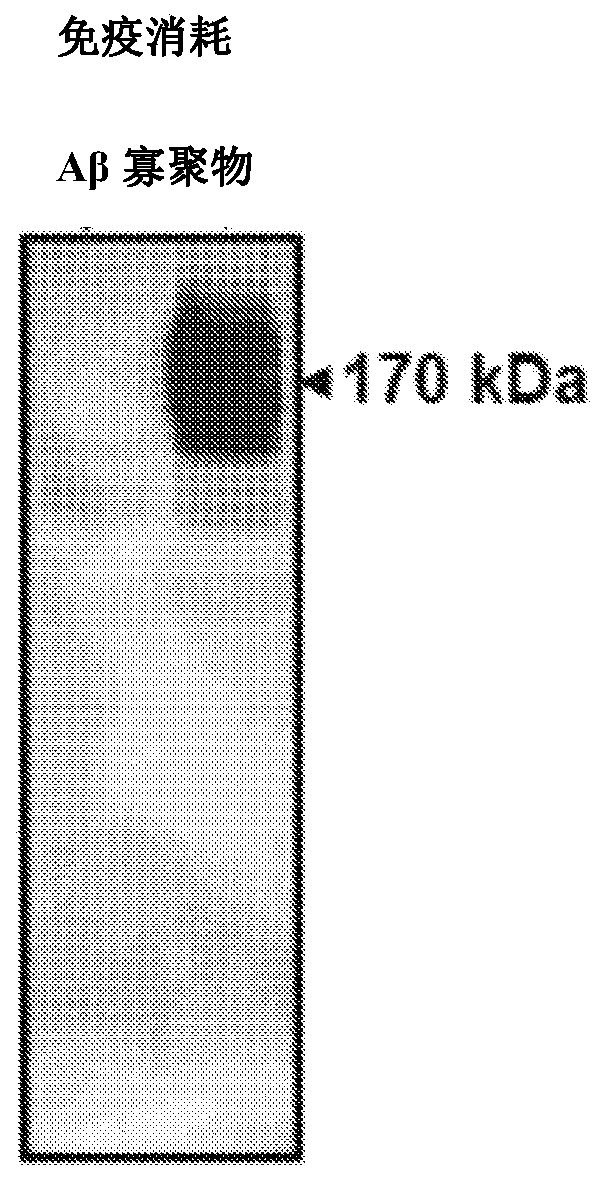
[0155] Example 2B: Using an amplification cycle that combines stages of incubation and physical disruption. Figure 2AThe samples in were incubated under circulating agitation (stirring at 500 RPM for 1 minute, then 29 minutes without shaking). Aggregation was measured over time by the binding of Thioflavin T (ThT) to amyloid fibrils using a flat plate spectrofluorometer (excitation: 435; emission: 485 nm). Graphs show mean and standard error of 3 replicates. The concentration of Aβ oligomers was estimated assuming an average molecular weight of 170 kDa. Figure 2B is a graph showing cycle-accelerated amplified amyloid formation measured by ThT fluorescence as a function of time seeded with various concentrations of the synthetic soluble oligomeric Aβ protein of Example 1. FIG. Under these conditions, the aggregation of monomeric Aβ1-42 protein induced with 8400, 300, 80 and 3 fmol of synthetic soluble misfolded Aβ protein was faster and easier to distinguish from the aggreg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com