Purification of bacterial polysaccharides
A polysaccharide, purification method technology, applied in bacteria, antibacterial drugs, chemical instruments and methods, etc., can solve the problems of infeasibility, long purification time, inability to expand the scale, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
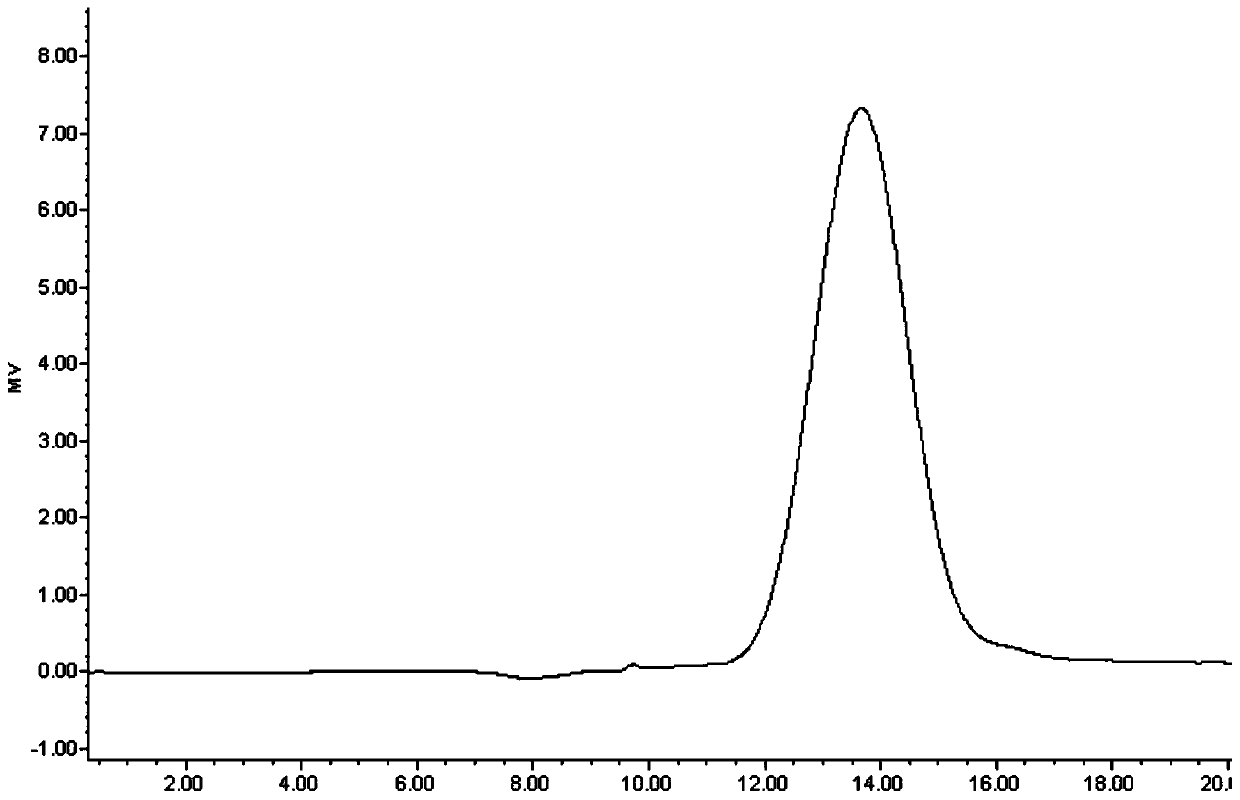
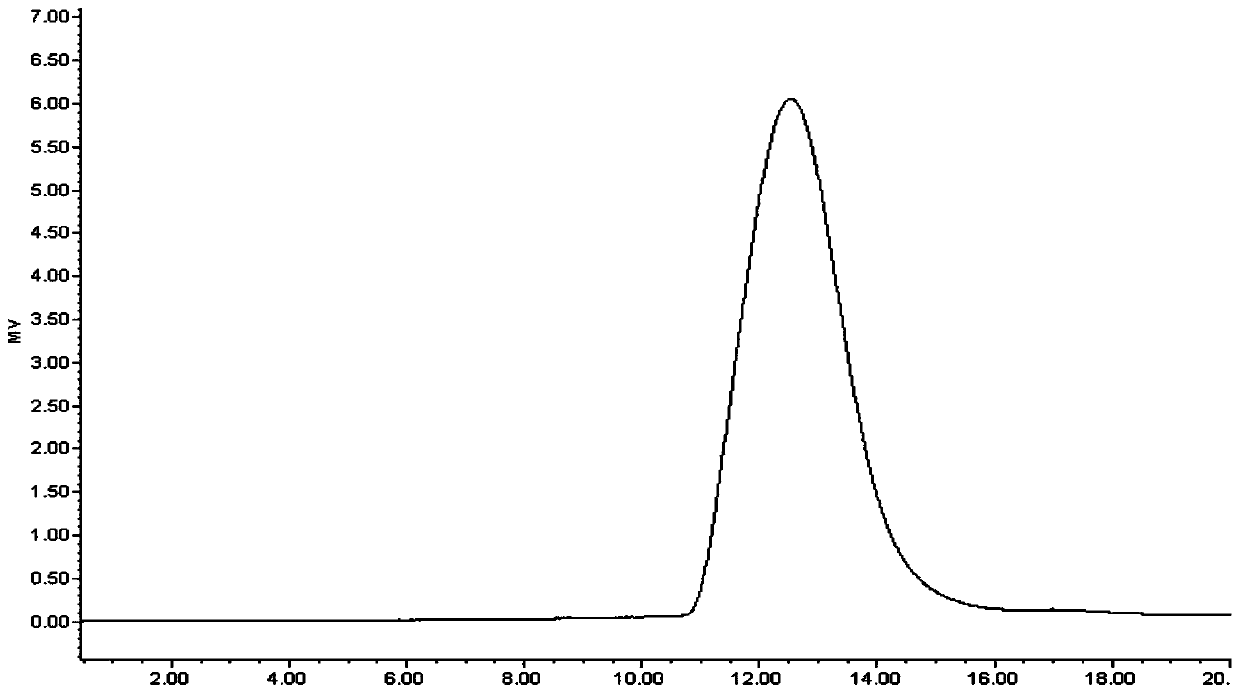
Image
Examples
Embodiment 1
[0044] Purification of MenW Polysaccharide (PS) Using Phenyl Sepharose
[0045] Fermentation broth (FB) was 100 kDa concentrated and diafiltered with 10-12 volumes of MilliQ water (MQW). After the above diafiltration and concentration, the polysaccharide was treated with 2M NaOH at 75±5°C for 2±0.5 hours. The PS was then cooled to room temperature. After cooling, the crude polysaccharide was concentrated at 100 kDa and diafiltered with 20 volumes of 20 mM Tris HCl (pH 8 ± 0.2). Thereafter, 20% w / v ammonium sulfate was added to the concentrated and diafiltered PS. It was then loaded onto phenyl sepharose resin using an XK16 / 20 column. The effluent was collected and then washed with equilibration buffer (20 mM Tris HCl (pH 8±0.2) and 20% ammonium sulfate) for 5-10 column volumes (CV). The collected material was then concentrated and diafiltered at 30 kDa with 20 volumes of MQW followed by 0.2 μ filtration.
Embodiment 2
[0047] Purification of MenW PS using sodium hydroxide (NaOH), ethanol, CTAB, sodium deoxycholate, sodium acetate
[0048] FB was 100 kDa concentrated and diafiltered with 10-12 volumes of MilliQ water (MQW). After diafiltration and concentration, partially purified PS was treated with 1 M NaOH at 75±5 °C for 2±0.5 h. The resulting PS was cooled to room temperature. After cooling, use 20 volumes of MQW to pass through a 100KDa PES membrane (0.1m 2 ) PS was concentrated and diafiltered, followed by ethanol precipitation using 100% v / v absolute ethanol and incubation overnight at 2-8°C. The next day, centrifugation was performed at 10550 xg, and the collected pellet was dissolved in MQW. 80% v / v absolute ethanol was added thereto, and stirred continuously at room temperature (25±2° C.) for 2±0.5 hours. Centrifugation was performed at 10550 x g, and the collected pellet was dissolved in MQW. It was then treated with 12% v / v of a 10% stock solution of cetyltrimethylammonium br...
Embodiment 3
[0050] Purify PS of MenW or MenY using NaOH treatment and carbon filtration:
[0051] With 100kDa (0.1m 2 ) PES membrane to concentrate and diafilter MenW or MenY fermentation broth. A 2.5 L working volume of fermentation broth was used in the purification process for N. meningitidis serogroup W and serogroup Y. The resulting MenW / MenY concentrate was subjected to alkaline treatment with 1±0.2M NaOH at 75±5°C for 2±0.5 hours. The resulting partially purified polysaccharide was then cooled to room temperature. After cooling, the partially purified PS was concentrated and diafiltered with 20 ± 2 volumes of MQW through a 100 KDa PES membrane to remove NaOH, followed by an MQW-filled Merck carbon filter ( Depth filter, 0.027m 2 ) for carbon filtration until OD 260nm ≤0.2. The collected filtrate was concentrated with a 100 kDa PES membrane and sterile filtered with a 0.2 μm PES module to obtain purified PS of MenW or MenY. The purified polysaccharides were stored at -20±2°C...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap