A method for culturing human umbilical cord blood mesenchymal stem cells
A technology for stem cells and human umbilical cord blood, applied in the field of cell biology, can solve the problems of separation and culture, high cost, unsuitable for large-scale production, etc. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The method for culturing human umbilical cord blood mesenchymal stem cells of the present embodiment comprises the following steps:
[0052] (1) Separation of mononuclear cells:
[0053] 1) The umbilical cord blood of premature infants was extracted under sterile conditions, and anticoagulated with heparin;
[0054] 2) Use D-Hanks balanced salt solution to dilute heparin to anticoagulate the umbilical cord blood in equal proportions;
[0055] 3) superimposing the diluted heparin anticoagulated umbilical cord blood on the Ficoll lymphocyte separation medium to obtain a mixed solution, the height ratio of the diluted heparin anticoagulated umbilical cord blood to the lymphocyte separation medium is 2:1;
[0056] 4) Centrifuge the mixture obtained in step 3) at 2000r / min for 20min, carefully absorb the white cloud layer at the interface,
[0057] Centrifuge with PBS balanced salt solution at 1000r / min to wash the precipitate twice.
[0058] (2) Culture of umbilical cord...
Embodiment 2
[0067] The method for culturing human umbilical cord blood mesenchymal stem cells of the present embodiment comprises the following steps:
[0068] (1) The isolation method of mononuclear cells is the same as in Example 1.
[0069] (2) Culture of umbilical cord blood mesenchymal stem cells in premature infants:
[0070] 1) adding the mononuclear cells isolated in step (1) to the first culture medium to make a single cell suspension, and counting the cells;
[0071] 2) According to the cell density 1×10 7 Individual / mL, the mononuclear cells in step 1) were inoculated in gelatin-coated T25 cell culture flasks;
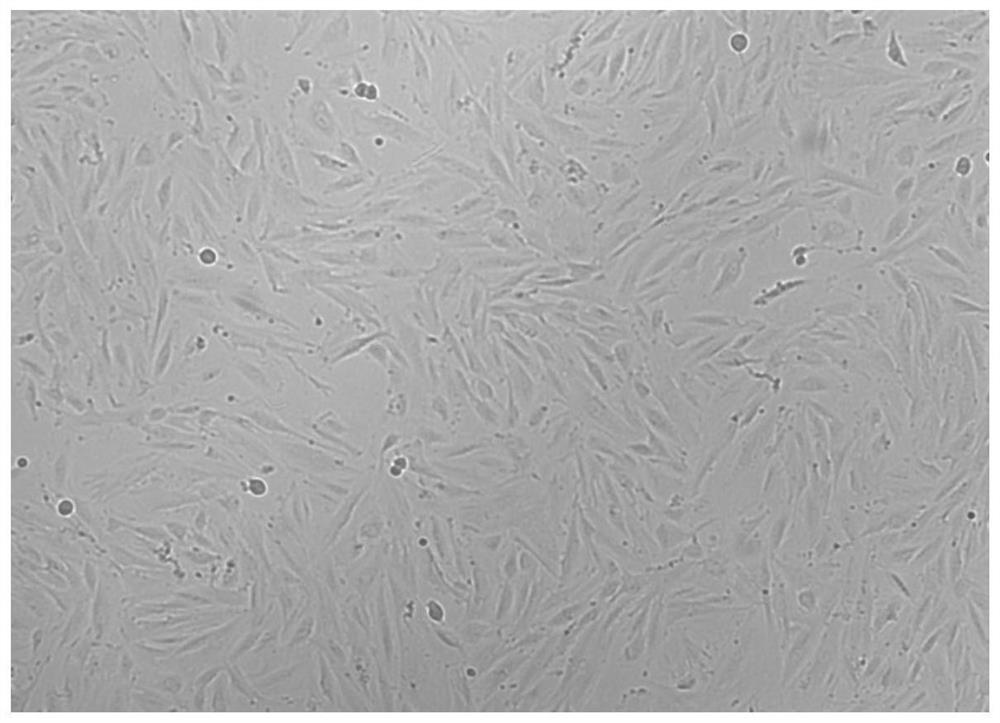
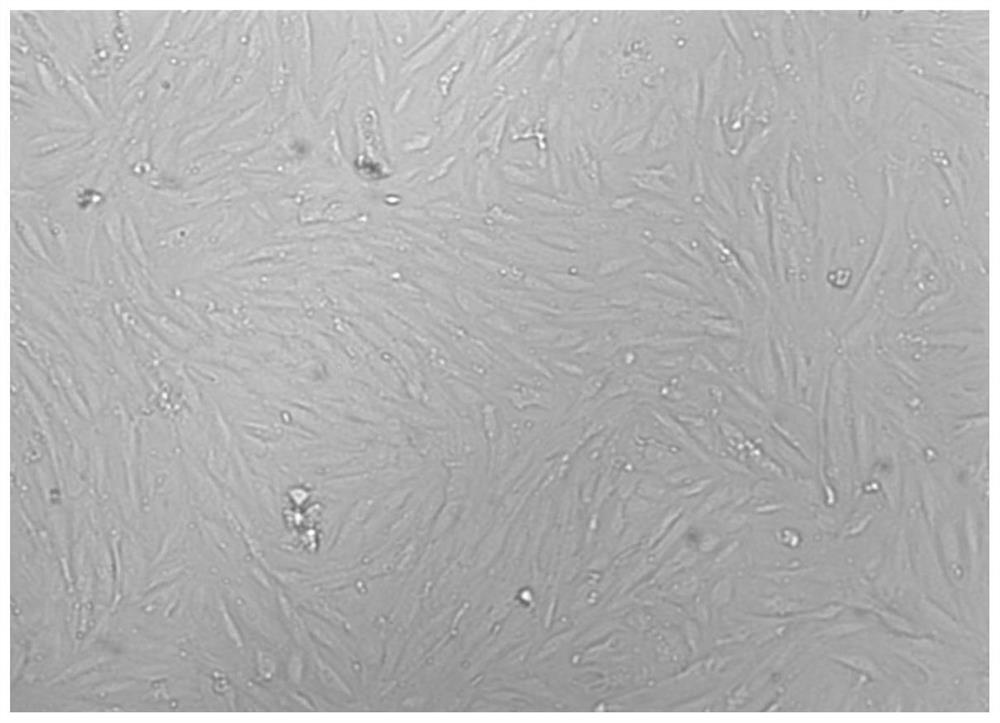
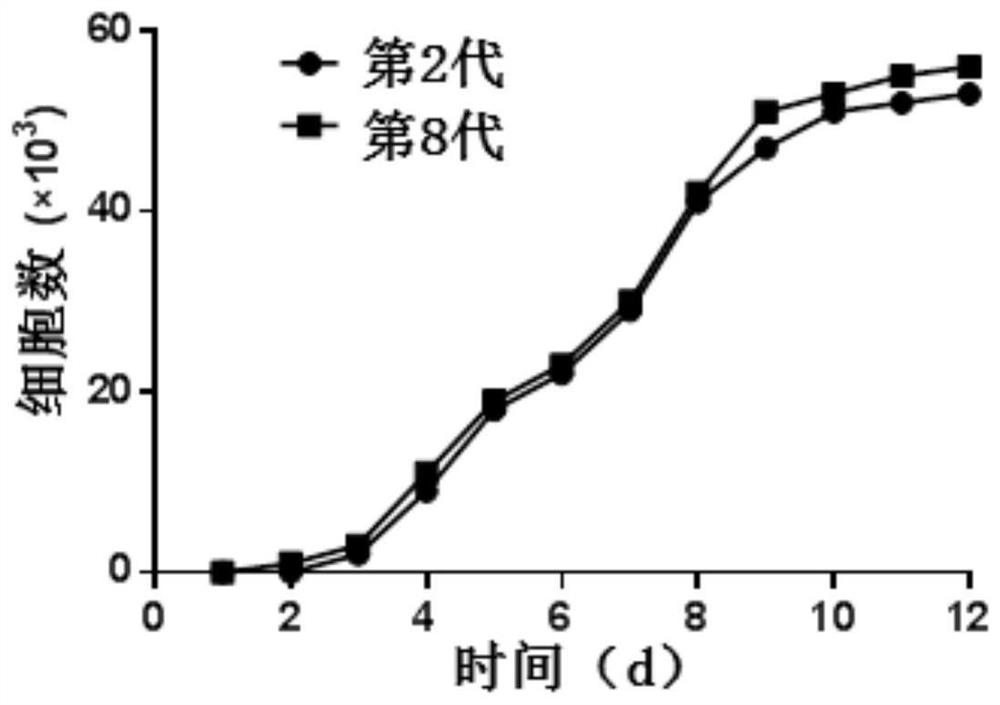
[0072] 3) Place the cell culture flask at 37°C with a volume fraction of 5% CO 2 Cultivate in a saturated humidity incubator, change the medium after 2 days, change the medium for the first time after 5-7 days, and change the medium every 3-4 days, and observe the growth and morphological characteristics of the primary cells every day under an inverted phase-contrast...
Embodiment 3
[0078] The method for culturing human umbilical cord blood mesenchymal stem cells of the present embodiment comprises the following steps:
[0079] (1) The isolation method of mononuclear cells is the same as in Example 1.
[0080] (2) Culture of umbilical cord blood mesenchymal stem cells in premature infants:
[0081] 1) adding the mononuclear cells isolated in step (1) to the first culture medium to make a single cell suspension, and counting the cells;
[0082] 2) According to the cell density 2×10 7Individual / mL, the mononuclear cells in step 1) were inoculated in gelatin-coated T25 cell culture flasks;
[0083] 3) Place the cell culture flask at 37°C with a volume fraction of 5% CO 2 Cultivate in a saturated humidity incubator, change the medium after 2 days, change the medium for the first time after 5-7 days, and change the medium every 3-4 days, and observe the growth and morphological characteristics of the primary cells every day under an inverted phase-contrast ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com