Molecular structure and synthesis method of new triple negative breast cancer drug
A triple-negative breast cancer and molecular structure technology, which is applied in drug combinations, antineoplastic drugs, and pharmaceutical formulations, can solve problems such as poor prognosis and achieve the effect of inhibiting tumor cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
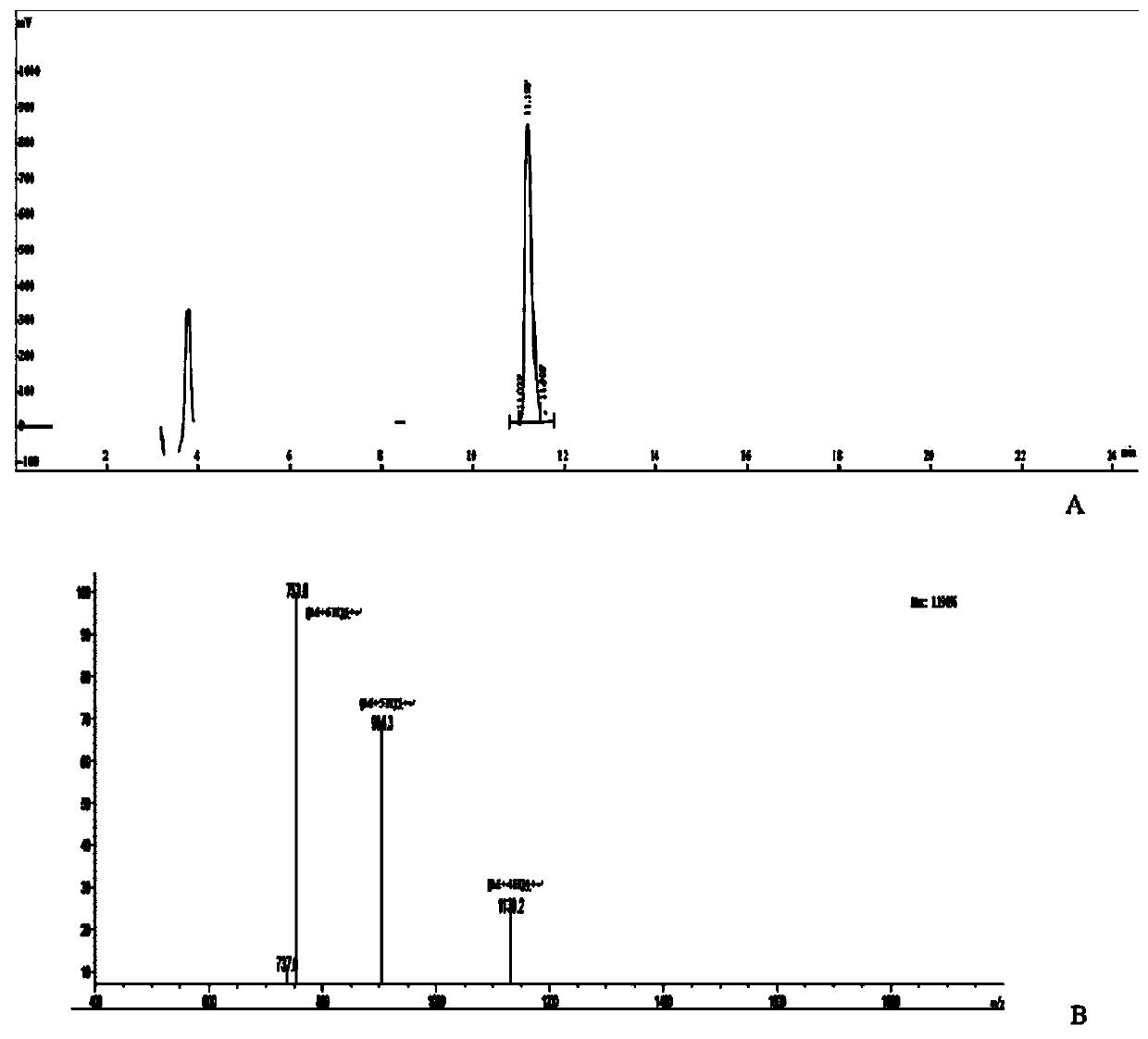
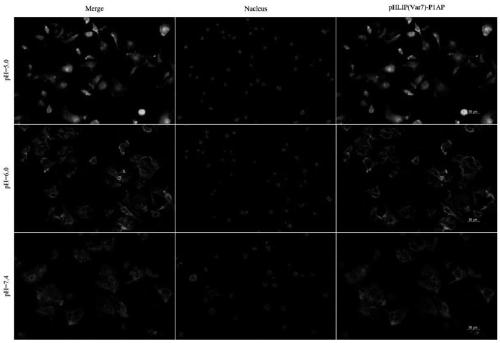
[0013] combined with Figure 1-4 , a molecular structure and a synthetic method of a novel anti-triple negative breast cancer drug, the molecular structure is pHLIP (Var7)-P1AP, wherein pHLIP (Var7)—AEEQNPWARYLEWLFPTETLLLELC, P1AP—CKKSRALF, and the synthetic method is as follows:
[0014] 1) Synthesize the pHLIP (Var7) Cys polypeptide sequence by solid-phase peptide synthesis: AEEQNPWARYLEWLFPTETLLLELC;
[0015] 2) Synthesize Cys P1AP polypeptide sequence by solid-phase peptide synthesis method: CKKSRALF;
[0016] 3) pHLIP(Var7)Cys and CysP1AP are linked by a disulfide bond to obtain the pHLIP(Var7)-P1AP sequence: AEEQNPWARYLEWLFPTETLLLELC-CKKSRALF.
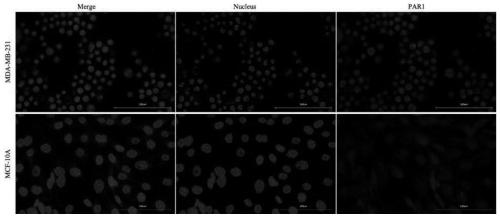
[0017] The present invention can specifically insert the C-terminus of pHLIP(Var7)-P1AP into triple-negative breast cancer cells, the disulfide bond is cleaved to release P1AP into tumor cells, and P1AP can target the intracellular part of tumor cell PAR1 receptor , to achieve the purpose of inhibiting tumor cell proliferation....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com