Method for manufacturing aromatic nitrile compound
A technology of a nitrile compound and a manufacturing method is applied in the fields of the manufacture of an aromatic carboxylic acid compound, 2-naphthaleneacetic acid, and the manufacture of 2-naphthaleneacetonitrile, which can solve the problems of insufficient yield and the like, and achieve high efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0317] Embodiment 1: the synthesis of carboxylic acid compound
[0318]
[0319] 3.00 g of 2'-naphthylethanone and 0.85 g of sulfur (1.5 mole times relative to 2'-naphthylethanone) were added to the reaction vessel after nitrogen replacement, and 4.61 g of morpholine (1.5 mole times relative to 2'-naphthyrophenone) was further added. 3 mole times of ethyl ketone), after stirring, react at 115° C. to 125° C. for 4 hours (to generate thioamide compound (thioamide compound)).
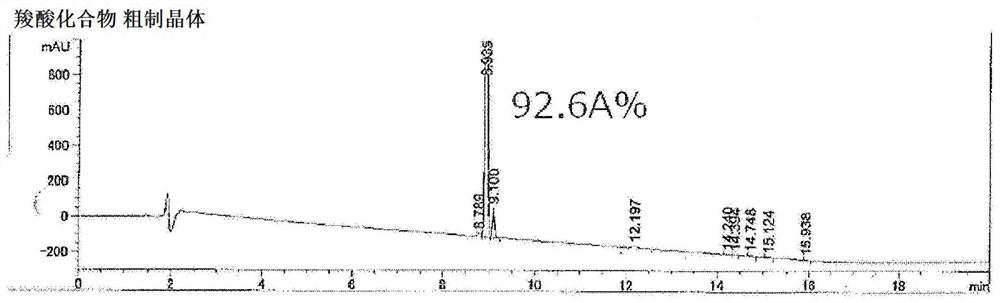
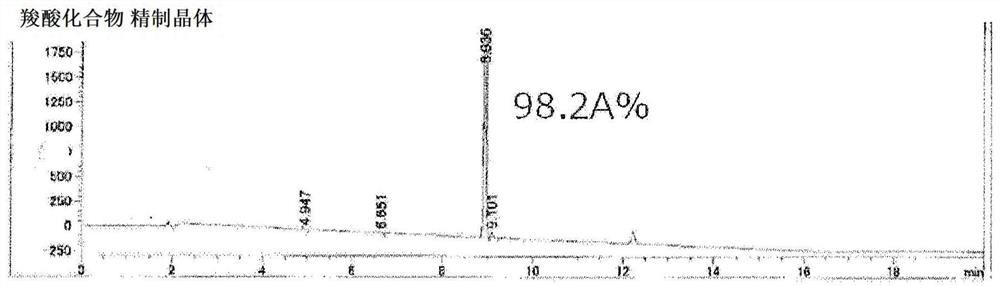
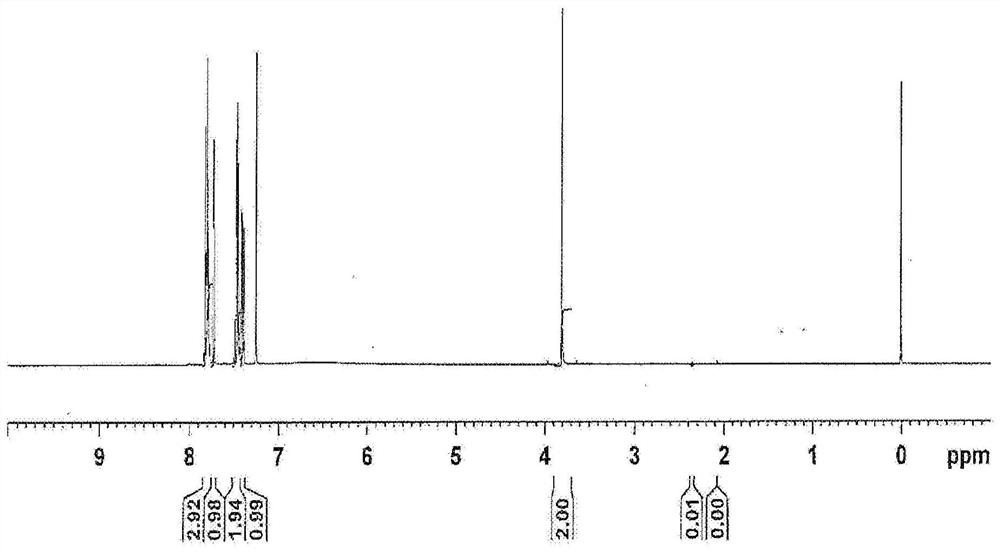
[0320] After cooling the reaction solution to 70°C to 80°C, add a 20% by weight aqueous solution of sodium hydroxide (a solution obtained by mixing 3.53 g of sodium hydroxide with 14.1 g of water. 5 moles relative to 2'-naphthylethanone twice the sodium hydroxide), react at 90°C to 105°C for 8 hours (hydrolysis). The reaction liquid was cooled to 50° C. to 60° C., 0.15 g of activated carbon (purified egret) was added, stirred, and then filtered. To the obtained filtrate, 35% hydrochloric acid (a solut...
Embodiment 2
[0324] Embodiment 2: the synthesis of carboxylic acid compound
[0325]
[0326] 120.00 g of 2'-naphthylethanone, 28.26 g of sulfur (1.25 mole times relative to 2'-naphthylethanone), p-toluenesulfonic acid monohydrate (p-Toluenesulfonic acid monohydrate) ) 13.41g (0.10 mole times relative to 2'-naphthylethanone) and morpholine 184.26g (3 mole times relative to 2'-naphthylethanone), after stirring, react at 115°C to 125°C for 9 hours (Generation of thioamide compounds).
[0327] After the reaction solution was cooled to 70°C to 80°C, an aqueous sodium hydroxide solution with a concentration of 20% by weight was added (a solution obtained by mixing 141.00 g of sodium hydroxide with 564.01 g of water. 5 moles relative to 2'-naphthylethanone twice the sodium hydroxide), react at 90°C to 105°C for 4 hours (hydrolysis). The reaction solution was cooled to 60°C to 70°C, 120.00 g of water and 240.00 mL of toluene were added, stirred at 65°C to 75°C, and after standing still, the ...
Embodiment 3
[0332] Embodiment 3: the synthesis of carboxylic acid compound
[0333]
[0334] 120.00 g of 2'-naphthylethanone, 120 mL of toluene (1.0 times the volume of 2'-naphthylethanone), and 0.34 g of methanesulfonic acid (1.0 times the volume of 2'-naphthylethanone 0.005 mol times), 184.26 g of morpholine (3 mol times relative to 2'-naphthylethanone) was further added, stirred, and then distilled for 15 hours.
[0335] After concentration, 28.26 g of sulfur (1.25 mole times relative to 2'-naphthylethanone) was added, and the mixture was reacted at 95°C to 105°C for 7 hours (to generate a thioamide compound).
[0336] After the reaction solution was cooled to 70°C to 80°C, an aqueous sodium hydroxide solution with a concentration of 20% by weight was added (a solution obtained by mixing 141.00 g of sodium hydroxide with 564.01 g of water. 5 moles relative to 2'-naphthylethanone twice the sodium hydroxide), react at 90°C to 105°C for 7 hours (hydrolysis). The reaction liquid was c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com