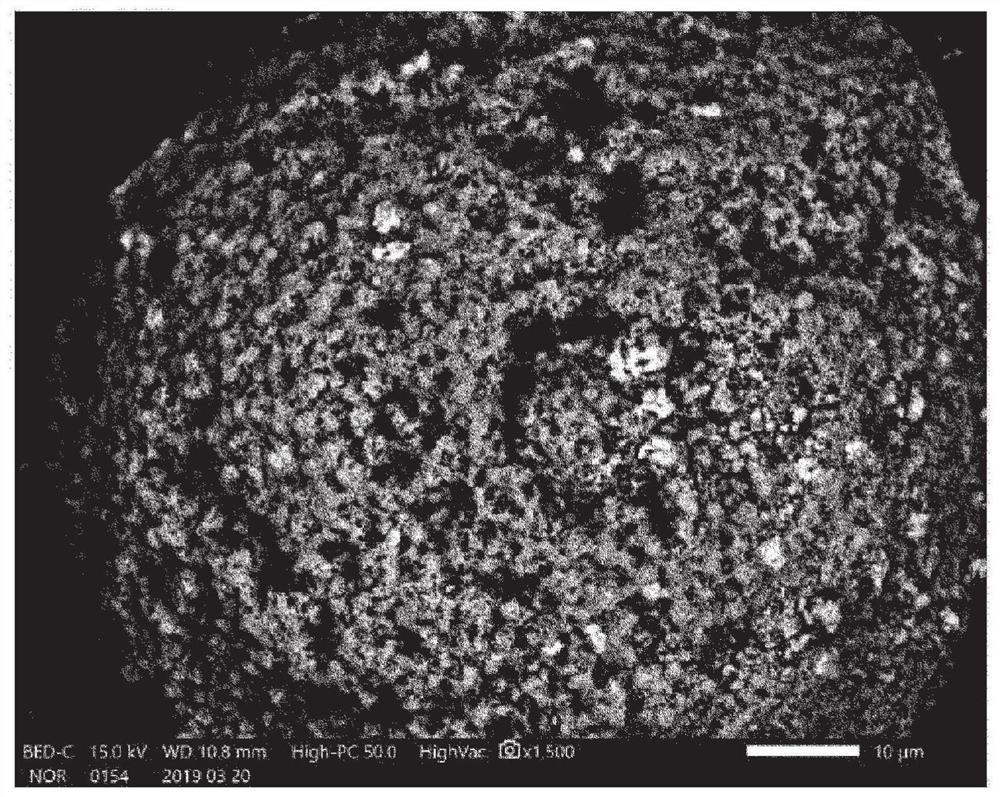
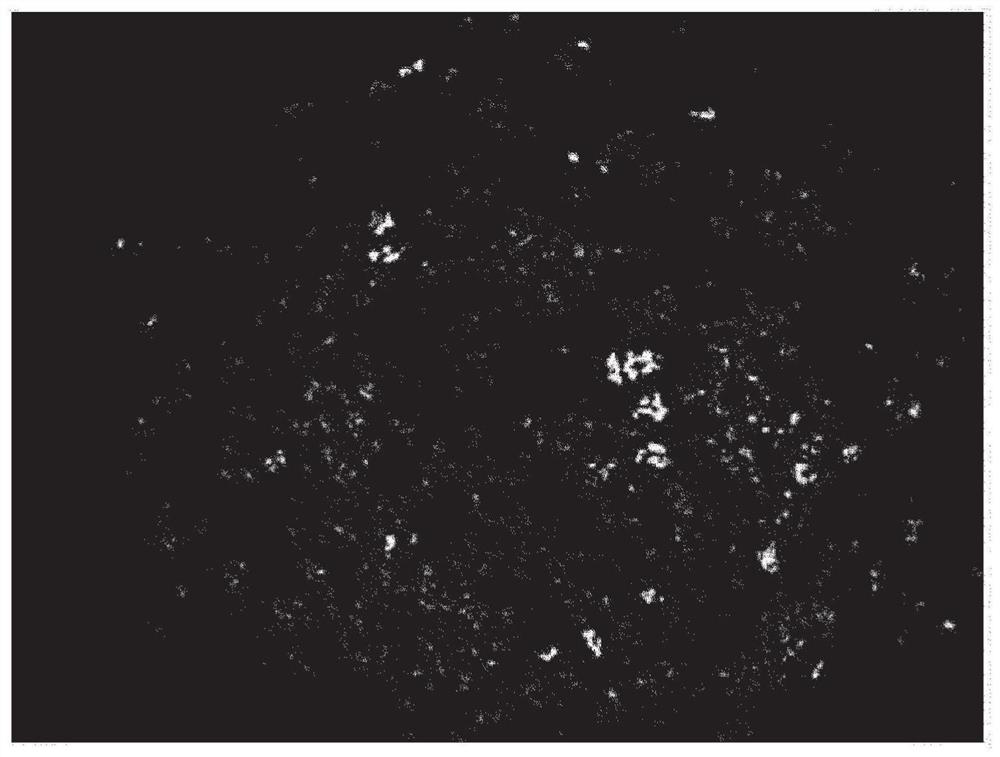
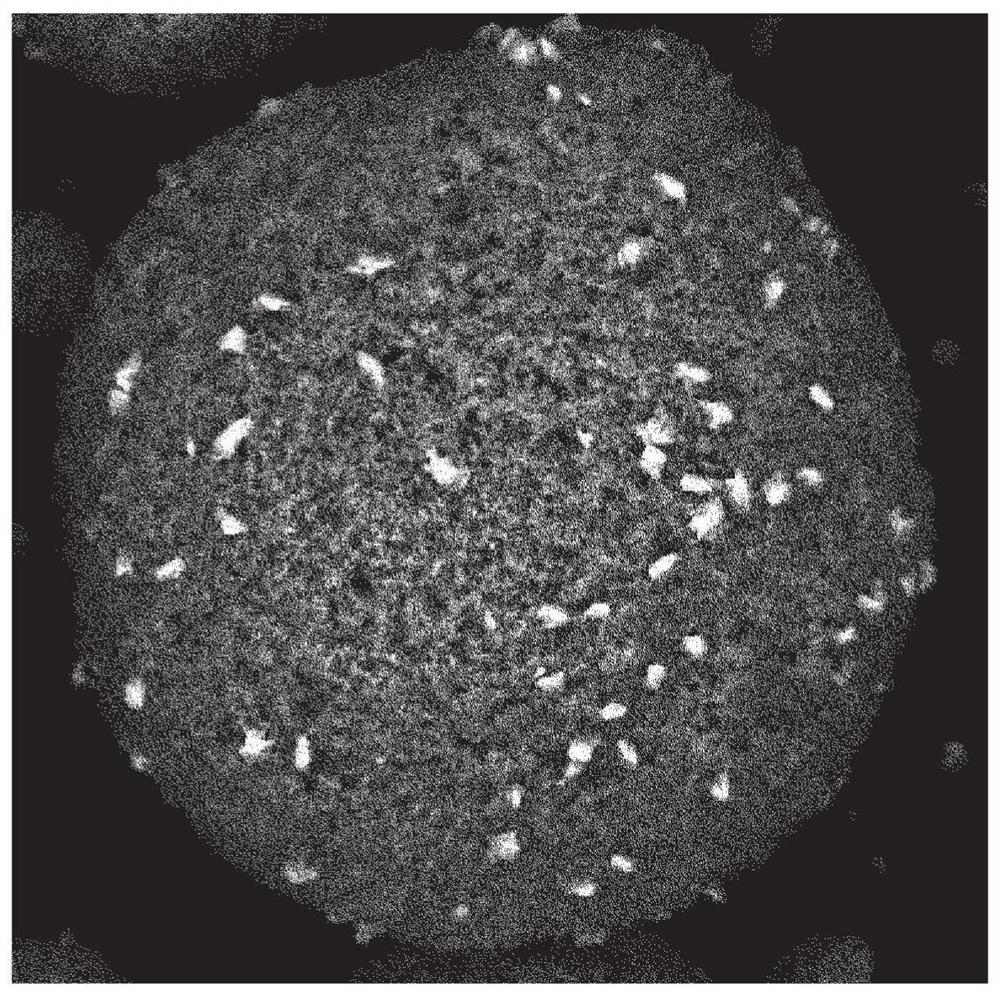
Dried granules for catalyst production, catalyst, and compound production method
A technology for drying particles and catalysts, applied in the direction of preparation of organic compounds, carbon-based compounds, heterogeneous catalyst chemical elements, etc., to achieve the effect of cheap yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0128] 800 parts by weight of ammonium heptamolybdate tetrahydrate was completely dissolved in 3040 parts by weight of pure water heated to 60°C. Next, 5.59 parts by weight of potassium nitrate was dissolved in 55 mL of pure water, and added to the above solution. Next, 274.6 parts by weight of iron nitrate nonahydrate, 571.5 parts by weight of cobalt nitrate hexahydrate, and 307.4 parts by weight of nickel nitrate hexahydrate were dissolved in 611 mL of pure water heated to 60°C. These solutions were mixed slowly while stirring. Next, 311.4 parts by weight of bismuth nitrate was added to an aqueous solution of nitric acid obtained by adding 79.3 parts by weight of nitric acid (60% by weight) to 330 mL of pure water, and the solution obtained by completely dissolving it was added to the above solution and carried out. Stir to combine. The slurry was dried by disc spray drying, and the obtained dry powder was precalcined while maintaining the maximum temperature of 440° C. fo...
manufacture example 2
[0134] 800 parts by weight of ammonium heptamolybdate tetrahydrate was completely dissolved in 3040 parts by weight of pure water heated to 60°C. Next, 11.0 parts by weight of cesium nitrate was dissolved in 100 mL of pure water, and added to the above solution. Next, 259.3 parts by weight of iron nitrate nonahydrate, 791.3 parts by weight of cobalt nitrate hexahydrate, and 109.8 parts by weight of nickel nitrate hexahydrate were dissolved in 615 mL of pure water heated to 60°C. These solutions were mixed slowly while stirring. Next, 311.4 parts by weight of bismuth nitrate was added to an aqueous solution of nitric acid obtained by adding 79.3 parts by weight of nitric acid (60% by weight) to 330 mL of pure water, and the solution obtained by completely dissolving it was added to the above solution and carried out. Stir to combine. The slurry was dried by disc spray drying, and the obtained dry powder was precalcined while maintaining the maximum temperature of 440° C. for ...
manufacture example 3
[0140] 800 parts by weight of ammonium heptamolybdate tetrahydrate was completely dissolved in 3040 parts by weight of pure water heated to 60°C. Next, 11.0 parts by weight of cesium nitrate was dissolved in 100 mL of pure water, and added to the above solution. Next, 259.3 parts by weight of iron nitrate nonahydrate, 769.3 parts by weight of cobalt nitrate hexahydrate, and 109.8 parts by weight of nickel nitrate hexahydrate were dissolved in 615 mL of pure water heated to 60°C. These solutions were mixed slowly while stirring. Next, 274.8 parts by weight of bismuth nitrate was added to an aqueous solution of nitric acid obtained by adding 70.0 parts by weight of nitric acid (60% by weight) to 300 mL of pure water, and the solution obtained by completely dissolving it was added to the above solution and carried out. Stir to combine. The slurry was dried by disc spray drying, and the obtained dry powder was precalcined while maintaining the maximum temperature of 440° C. for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com