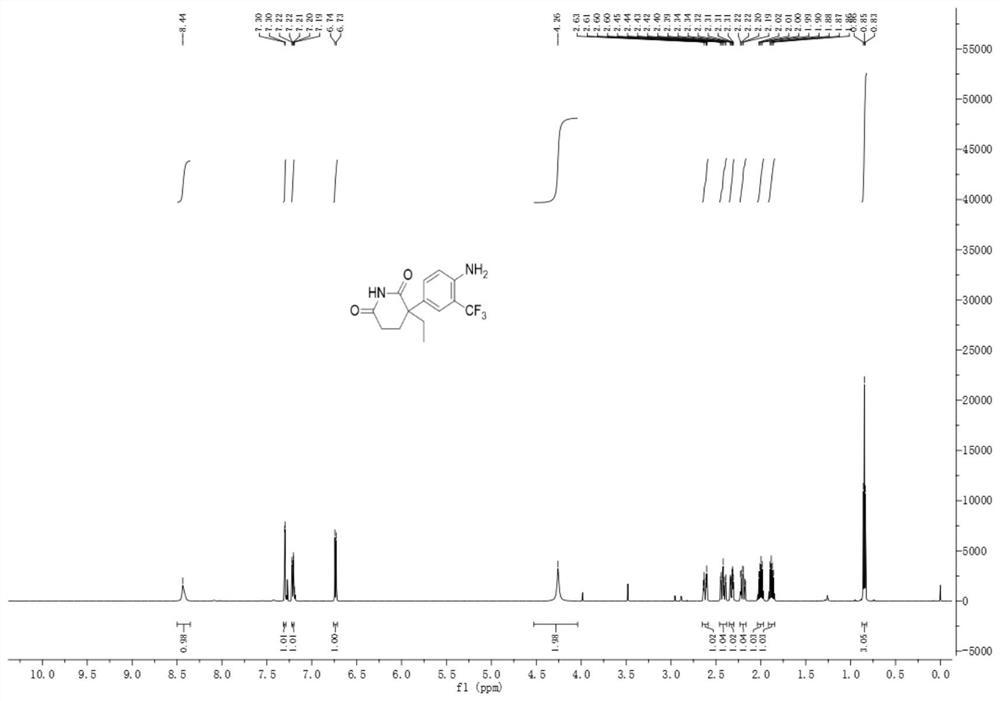
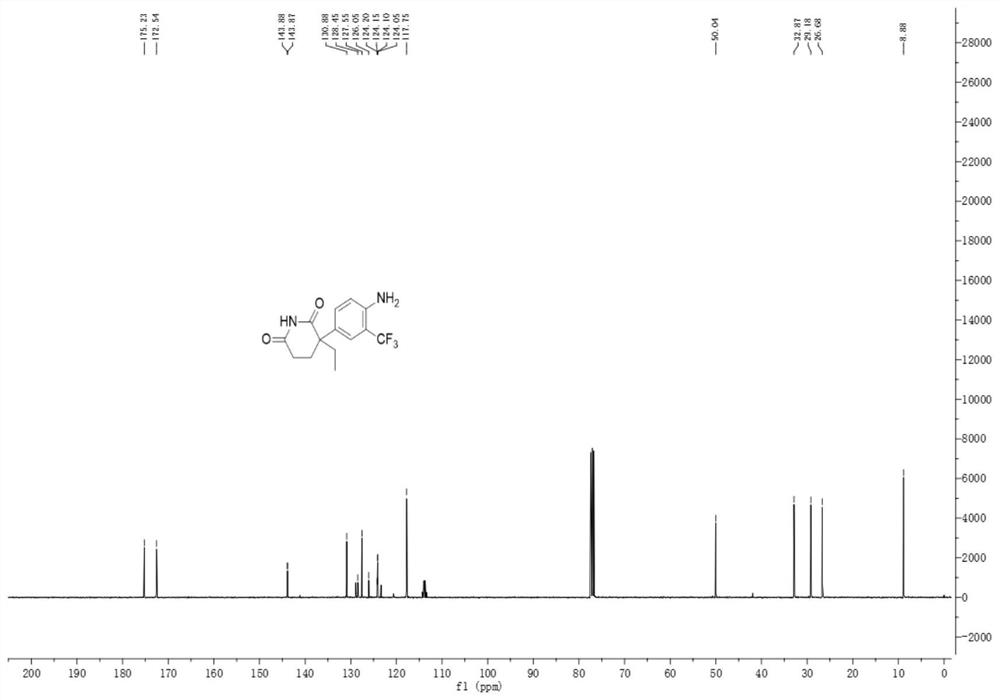
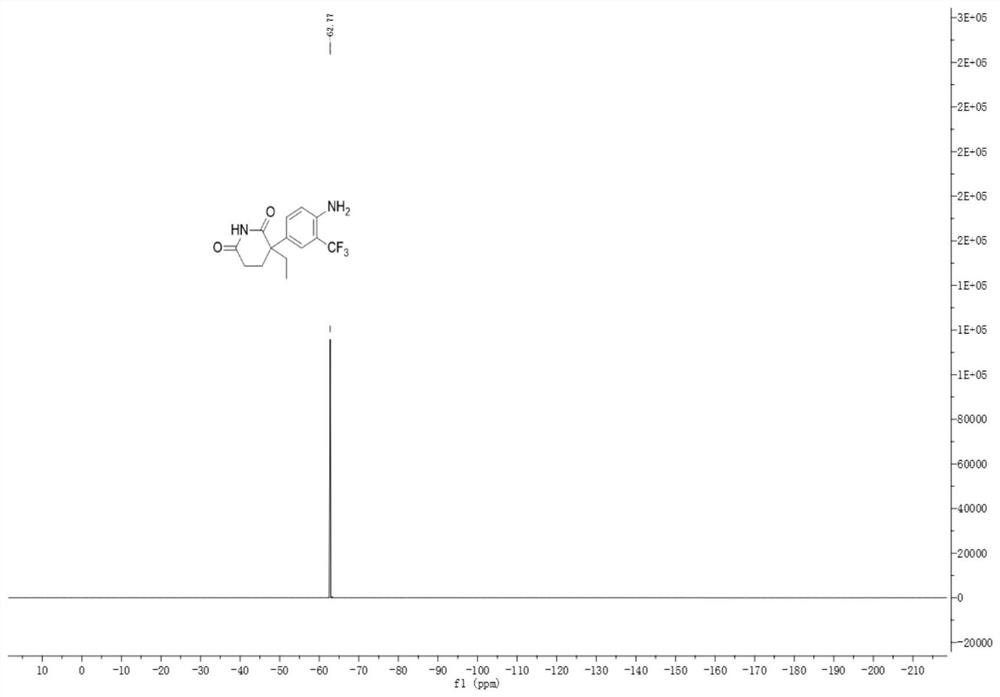
A kind of trifluoromethylated aniline compound and application thereof
A technology for trifluoromethylated aniline and compounds, which is applied in the field of trifluoromethylated aniline compounds, can solve the problems of uncertain action, low yield, increased research cost and difficulty, and achieve mild reaction conditions, high reaction efficiency, The direct effect of the trifluoromethylation modification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Add 46.4mg (0.2mmol) of aminoglutethimide to a mixed solvent of 2ml of DMF and water (1ml:1ml), add 126.4mg (0.4mmol) of 1-(trifluoromethyl)-1,2-benzene Iodosyl-3(1H)-one, 13.3mg (0.04mmol) fluorescein, reacted under blue light irradiation at room temperature for 6 hours, after the reaction, extraction operation: 1. Add saturated NaCl aqueous solution and acetic acid to the reaction solution in sequence Ethyl was extracted twice. 2. Add 50 mL of saturated NaCl aqueous solution and 30 mL of ethyl acetate for the first time to perform one extraction. 3. After the first extraction, collect the organic layer, take the aqueous phase layer and add 20 ml of ethyl acetate for the second extraction. After the extraction was completed, the organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation at room temperature to obtain the crude compound. The crude compound was subjected to silica gel column chromatograph...
Embodiment 2
[0039] In addition to changing the mass and moles of 1-(trifluoromethyl)-1,2-phenyliodyl-3(1H)-one to 63.2 mg (0.2 mmol), the mass and moles of fluorescein to 0.664 mg (0.002mmol), other operation is constant, and operating procedure is with embodiment 1, obtains the compound pure product 12mg shown in formula (Ia), and reaction yield is 20%.
Embodiment 3
[0041] In addition to changing the mass and moles of 1-(trifluoromethyl)-1,2-phenyliodyl-3(1H)-one to 189.6 mg (0.6 mmol), the mass and moles of fluorescein to 33.2 mg (0.1mmol), other operation is constant, and operating procedure is with embodiment 1, obtains the compound pure product 15mg shown in formula (Ia), and reaction yield is 25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com