Tryptophan 7-bit maleimide cyclized derivative as well as preparation method and application thereof
A technology of maleimide and tryptophan, which is applied in the preparation methods of peptides, chemical instruments and methods, drug combinations, etc., can solve the problem that the C-7 position cyclization reaction cannot be realized, the reaction conditions are severe, and the reaction yield is low problems, to achieve the effect of good anti-tumor application prospect, high site selectivity, and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Compound (I a ) preparation
[0047]
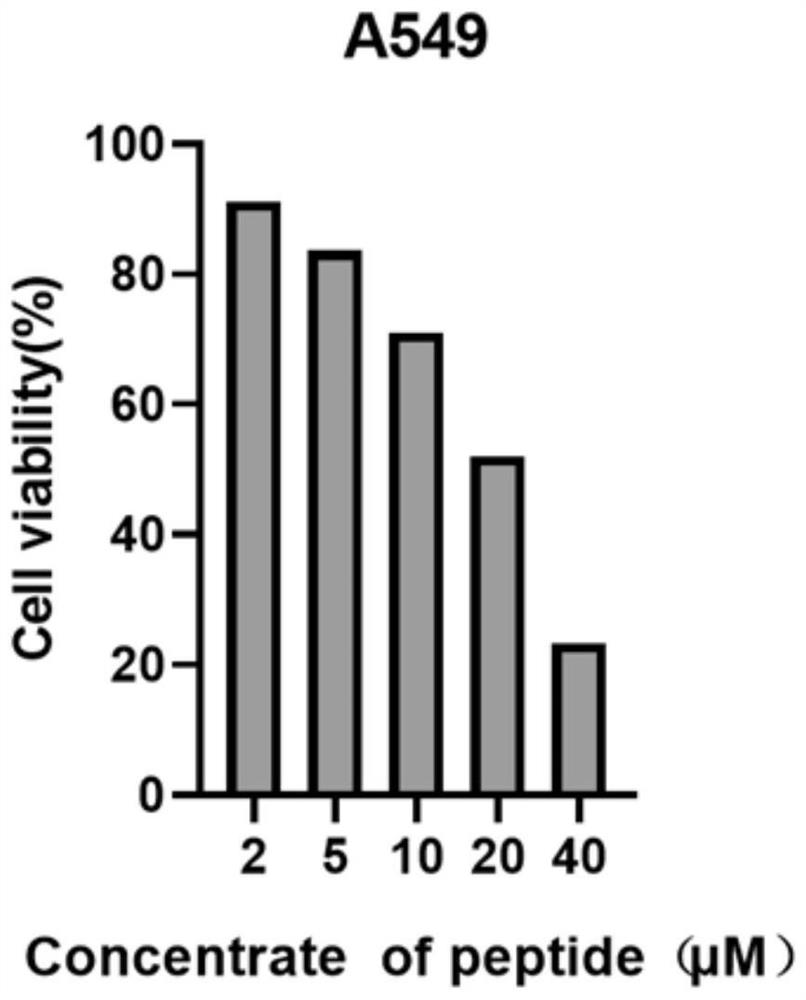
[0048] as formula II a Indicated maleimide-containing tryptophan linear polypeptide (general synthetic procedure for cyclic peptide precursor (II)) (67.84 mg, 0.12 mmol), [RhCp*Cl 2 ] 2 (7.4 mg, 0.012 mmol) suspended in 10 mL DCM, then AgNTf was added 2 (18.6 mg, 0.048 mmol) and silver oxide (41.7 mg, 0.18 mmol). The tube was sealed and the mixture was heated to 80°C for 24 hours. Saturated NaCl aqueous solution was added to the reaction mixture, extracted with ethyl acetate, the organic layer was taken, dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation at room temperature to obtain the crude product; further purified by silica gel column or preparative silica gel plate (dichloromethane The volume ratio to methanol is 15:1; Rf=0.4), the solvent is collected and spin-dried to obtain formula I a The compound shown is pure 15.0 mg, yield 22%, compound I a Antitumor ac...
Embodiment 2
[0050] Example 2: Compound (I b ) preparation
[0051]
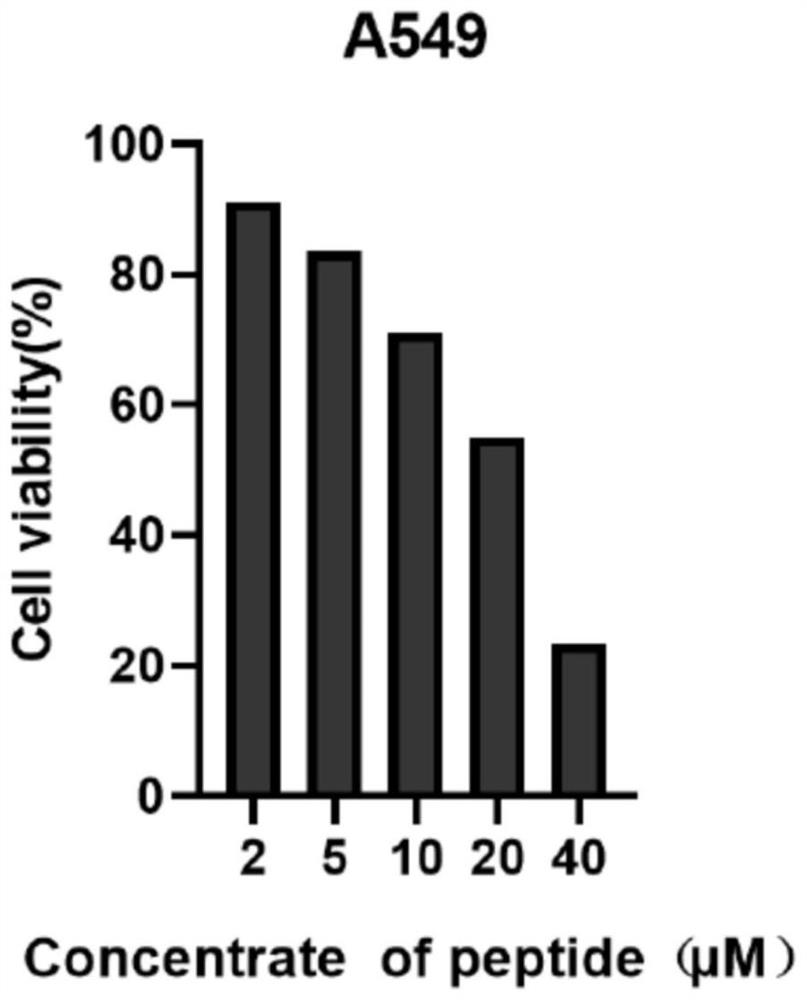
[0052] as formula II b The indicated maleimide-containing tryptophan linear polypeptide (general synthetic procedure for cyclic peptide precursor (II)) (74.89 mg, 0.12 mmol), [RhCp*Cl 2 ] 2 (7.4 mg, 0.012 mmol) suspended in 10 mL DCM, then AgNTf was added 2 (18.6 mg, 0.048 mmol) and silver oxide (41.7 mg, 0.18 mmol). The tube was sealed and the mixture was heated to 80°C for 24 hours. Saturated NaCl aqueous solution was added to the reaction mixture, extracted with ethyl acetate, the organic layer was taken, dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation at room temperature to obtain the crude product; further purified by silica gel column or preparative silica gel plate (dichloromethane The volume ratio to methanol is 10:1; Rf=0.3), the solvent is collected and spin-dried to obtain formula I b The compound shown was 11.3 mg of pure product, and the yield was 15%...
Embodiment 3
[0054] Example 3: Compound (I c ) preparation
[0055]
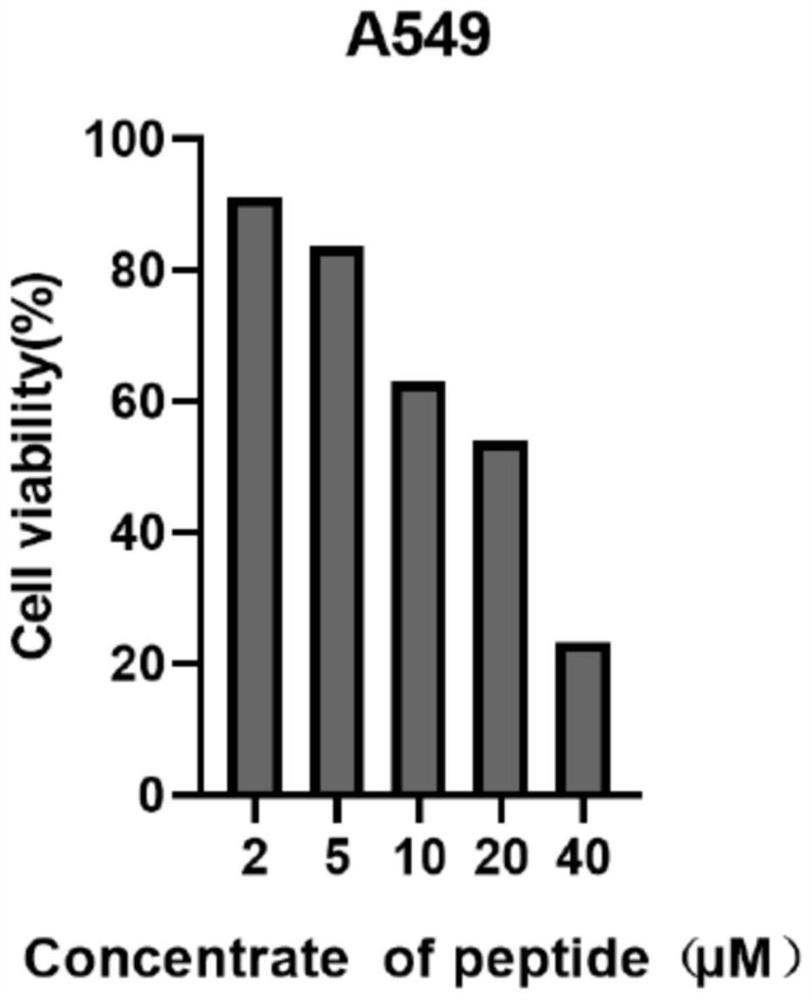
[0056] as formula II c The maleimide-containing tryptophan linear polypeptide shown (general synthetic procedure for cyclic peptide precursor (II)) (114.66 mg 0.12 mmol), [RhCp*Cl 2 ] 2 (7.4 mg, 0.012 mmol) suspended in 10 mL DCM, then AgNTf was added 2 (18.6 mg, 0.048 mmol) and silver oxide (41.7 mg, 0.18 mmol). The tube was sealed and the mixture was heated to 80°C for 24 hours. Saturated NaCl aqueous solution was added to the reaction mixture, extracted with ethyl acetate, the organic layer was taken, dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation at room temperature to obtain the crude product; further purified by silica gel column or preparative silica gel plate (dichloromethane The volume ratio to methanol is 20:1; Rf=0.23), the solvent is collected and spin-dried to obtain formula I c The compound shown was 11.23 mg of pure product, and the yield was 35%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com