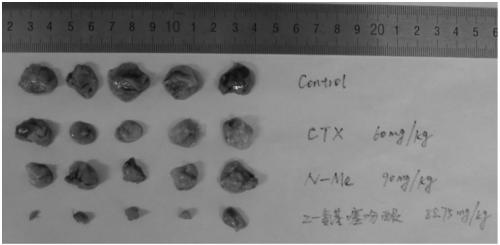
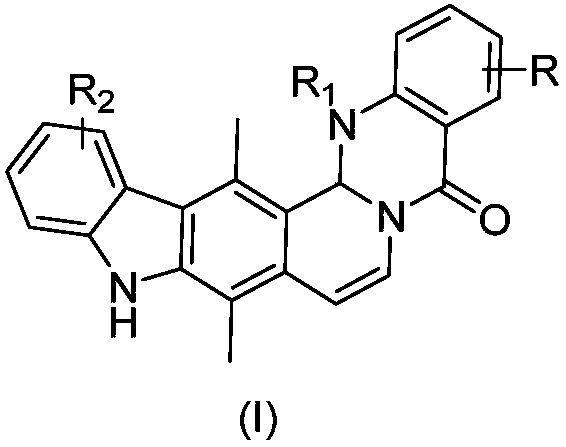
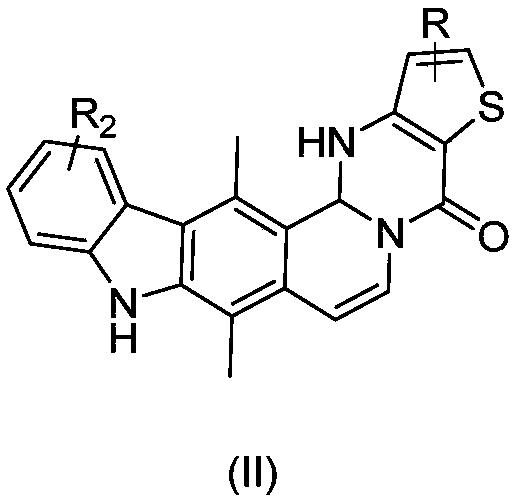
Ellipticine derivatives, drug composition, preparation method and application thereof
A technology of ellipticine and derivatives, applied in the field of medicine, can solve problems such as drug resistance and insensitivity of tumor stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] 9,15-Dimethyl-15b,16-dihydroquinazolino[3',2':1,2]pyrido[4,3-b]carbazol-5(10H)-one
[0085] 9,15-Dimethyl-15b,16-dihydroquinazolino[3’,2’:1,2]pyrido[4,3-b]carbazol-5(10H)-one
[0086]
[0087] Dissolve 25 mg (0.1 mmol) of ellipticine and 27 mg (0.2 mmol) of 2-aminobenzoic acid in 3 ml of anhydrous N,N-dimethylformamide, and add 38 mg (0.2 mmol) of 1-ethyl-(3- Dimethylaminopropyl) carbodiimide hydrochloride (EDCI), reacted at room temperature for 5 hours, a yellow solid precipitated, and TLC monitoring showed that the reaction was complete. After concentration, the residue was extracted with dichloromethane (20 mL×3), and the combined organic phases were sequentially washed with saturated sodium bicarbonate solution, purified water and saturated sodium chloride solution, and dried overnight over anhydrous sodium sulfate. After evaporating dichloromethane, the obtained yellow solid was treated with absolute ethanol to obtain 20 mg of a yellow solid product with a yiel...
Embodiment 2
[0089] 3,9,15-Trimethyl-15b,16-dihydroquinazolino[3',2':1,2]pyrido[4,3-b]carbazol-5(10H)-on e
[0090] 3,9,15-Trimethyl-15b,16-dihydroquinazolino[3’,2’:1,2]pyrido[4,3-b]carbazol-5(10H)-one
[0091]
[0092] Dissolve 25 mg (0.1 mmol) of ellipticine and 30 mg (0.2 mmol) of 2-amino-5-methylbenzoic acid in 3 ml of anhydrous N,N-dimethylformamide, add 38 mg (0.2 mmol) of EDCI, and After 5 hours of reaction, a yellow solid precipitated out, and TLC monitoring showed that the reaction was complete. After concentration, the residue was extracted with dichloromethane (20 mL×3), and the combined organic phases were sequentially washed with saturated sodium bicarbonate solution, purified water and saturated sodium chloride solution, and dried overnight over anhydrous sodium sulfate. After evaporating dichloromethane, the obtained yellow solid was treated with absolute ethanol to obtain 24 mg of a yellow solid product with a yield of 63.3%. 1 H NMR (500MHz, DMSO-d 6)δ11.30(s,1H,N-H)...
Embodiment 3
[0094] 4,9,15-Trimethyl-15b,16-dihydroquinazolino[3',2':1,2]pyrido[4,3-b]carbazol-5(10H)-one
[0095] 4,9,15-Trimethyl-15b,16-dihydroquinazolino[3’,2’:1,2]pyrido[4,3-b]carbazol-5(10H)-one
[0096]
[0097] Dissolve 25mg (0.1mmol) of ellipticine and 30mg (0.2mmol) of 2-amino-6-methylbenzoic acid in 3ml of anhydrous N,N-dimethylformamide, add 38mg (0.2mmol) of EDCI, and After 5 hours of reaction, a yellow solid precipitated out, and TLC monitoring showed that the reaction was complete. After concentration, the residue was extracted with dichloromethane (20 mL×3), and the combined organic phases were sequentially washed with saturated sodium bicarbonate solution, purified water and saturated sodium chloride solution, and dried overnight over anhydrous sodium sulfate. After evaporating dichloromethane, the obtained yellow solid was treated with absolute ethanol to obtain 17 mg of a yellow solid product with a yield of 44.9%. 1 H NMR (500MHz, DMSO-d 6 )δ11.27(s, 1H, N-H), 8.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com