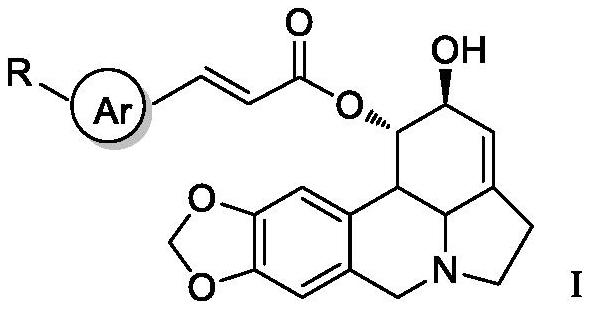
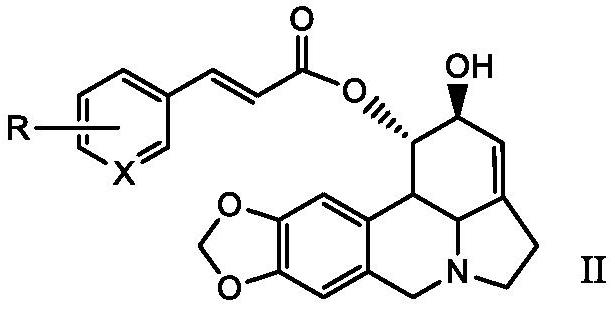
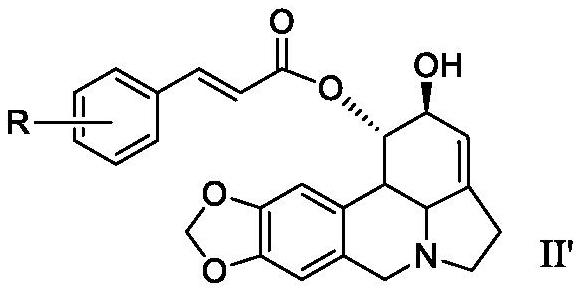
A kind of lycorine β-aryl acrylate derivative and its preparation method and application
A technology of aryl acrylate and lycorine, applied in the field of biomedicine, can solve the problems of large cell damage and exacerbation of the disease, and achieve good anti-tumor application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 : 2-tert-butyl dimethyl silicide-stone garlic (INB)
[0098] INB structure:
[0099] Standard (100.0 mmol) and IM (150.0 mmol) were dissolved in 300 ml of DMF in a 2000 ml round bottom reaction flask, and TBSCl (150.0 mmol) was slowly added with vigorous stirring. The temperature rise to 40 ° C for complete dissolved reaction system, after stirring for 4 h, HPLC tracking detects that the starting material stone residual remaining amount is less than 5%. To the reaction system, 500 ml of ethyl acetate and 800 ml of purified water were sequentially added, and the mixture was stirred at room temperature for 10 minutes. Concentrated, column chromatography isolated purified white solid INB. 1 HNMR (500MHz, CDCL 3 Δ6.80 (S, 1H), 6.56 (S, 1H), 5.89 (D, J = 1.1 Hz, 2H), 5.39 (S, 1H), 4.25 (S, 1H), 4.25 (S, 1H), 4.10 (D, J = 14.0 Hz, 1H), 3.45 (D, J = 14.0 Hz, 1H), 3.38-3.25 (M, 1H), 2.78 (D, J = 10.6Hz, 1H), 2.69 (D, J = 10.6Hz, 1H), 2.59 (DD, J = 15.9, 7.9 Hz, 2H), 2.3...
Embodiment 2
[0100] Example 2 : 1- (2-fluoriniacinyl) - stone garine (S1)
[0101] S1 structure:
[0102] A: 250ml round bottom reaction flask INB (10.0 mmol) and 2-fluorinic acid (12.0 mmol) in 50 ml of dichloromethane (12.0 mmol) were dissolved in 50 mL of dichloromethane in room temperature nitrogen. EDCI.HCl (18.0 mmol), DMAP (1.0 mmol) was added sequentially. After completing the reaction at 35 ° C, the HPLC tracking detection INB remaining is less than 8%. The reaction solution was washed once with 100 ml of purified water, 10% sodium chloride solution 100 ml each, and then concentrated under reduced pressure.
[0103] B: 250 ml of round bottom reaction flask was dissolved in 50 ml of anhydrous ethanol, and 5.0 ml of concentrated hydrochloric acid was slowly added dropwise. The reaction was stirred at 80 ° C for 1.0 h, and the heating was closed naturally 50 ° C, 5.0 ml of ammonia water was slowly added dropwise. 80 ml of ethyl acetate and 80 ml of purified water were sequentially adde...
Embodiment 3
[0104] Example 3 : 1- (3-fluoriniacinyl) - stone garine (S2)
[0105] S2 structure:
[0106] A: 250ml round bottom reaction flask INB (10.0 mmol) and 3-fluorinic acid (12.0 mmol) in 50 ml of dichloromethane (12.0 mmol) were dissolved in 50 mm of dichloromethane in room temperature nitrogen. EDCI.HCl (18.0 mmol), DMAP (1.0 mmol) was added sequentially. After completing the reaction at 35 ° C, the HPLC tracking detection INB remaining is less than 8%. The reaction solution was washed once with 100 ml of purified water, 10% sodium chloride solution 100 ml each, and then concentrated under reduced pressure.
[0107] B: 250 ml of round bottom reaction flask was dissolved in 50 ml of anhydrous ethanol, and 5.0 ml of concentrated hydrochloric acid was slowly added dropwise. The reaction was stirred at 80 ° C for 1.0 h, and the heating was closed naturally 50 ° C, 5.0 ml of ammonia water was slowly added dropwise. 80 ml of ethyl acetate and 80 ml of purified water were sequentially adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com