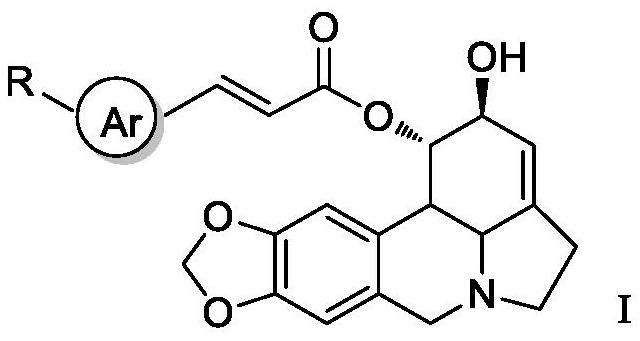
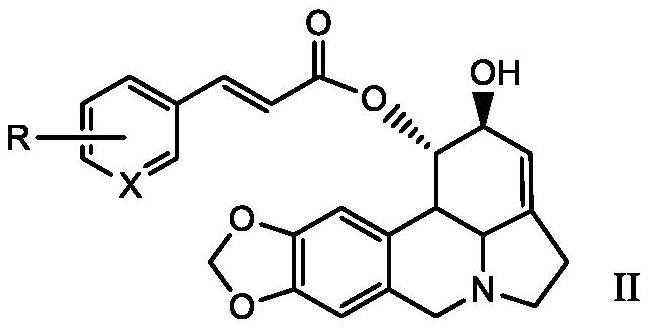
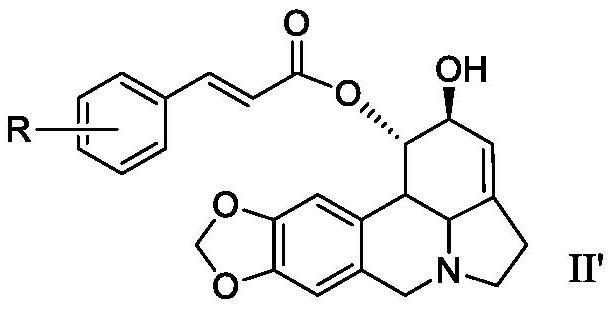
Lycorine beta-aryl acrylate derivative as well as preparation method and application thereof
A technology of aryl acrylate and lycorine, applied in the field of biomedicine, can solve the problems of large cell damage and exacerbation of the disease, and achieve good anti-tumor application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 : 2-tert-butyldimethylsilyl-lycorine (INB)
[0098] INB structure:
[0099] Lycorine (100.0mmol) and Im (150.0mmol) were dissolved in 300ml DMF in a 2000ml round bottom reaction flask, and TBSCl (150.0mmol) was slowly added under vigorous stirring at room temperature. Raise the temperature to 40° C. to completely dissolve the reaction system. After 4 hours of heat preservation and stirring, HPLC traces and detects that the residual amount of lycorine in the starting material is less than 5%. Add 500ml of ethyl acetate and 800ml of purified water to the reaction system in sequence, stir at room temperature for 10 minutes, then separate the liquid, wash the organic phase with 500ml of 10% sodium chloride solution, dry the liquid with 100.0g of anhydrous sodium sulfate, filter, and reduce pressure Concentration, separation and purification by column chromatography gave INB as a white solid. 1 HNMR (500MHz, CDCl 3 )δ6.80(s,1H),6.56(s,1H),5.89(d,J=1.1Hz,2H),5...
Embodiment 2
[0100] Example 2 : 1-(2-fluorocinnamoyl)-lycorine (S1)
[0101] S1 structure:
[0102] a: Dissolve 2-tert-butyldimethylsilyl-lycorine INB (10.0mmol) and 2-fluorocinnamic acid (12.0mmol) in 50ml dichloromethane in a 250ml round-bottomed reaction flask, and put it under nitrogen protection at room temperature EDCI.HCl (18.0 mmol), DMAP (1.0 mmol) were added sequentially. After the addition is complete, heat-preserve and stir the reaction at 35° C., and post-process after detecting that the remaining amount of INB is less than 8% by HPLC. The reaction liquid was washed with 100 ml of purified water and 100 ml of 10% sodium chloride solution in turn, concentrated under reduced pressure and then used for later use.
[0103] b: Dissolve the above product in 50 ml of absolute ethanol in a 250 ml round-bottomed reaction bottle, and slowly add 5.0 mL of concentrated hydrochloric acid dropwise with stirring at room temperature. After the dropwise addition, stir and react at 80°C ...
Embodiment 3
[0104] Example 3 : 1-(3-fluorocinnamoyl)-lycorine (S2)
[0105] S2 structure:
[0106] a: Dissolve 2-tert-butyldimethylsilyl-lycorine INB (10.0mmol) and 3-fluorocinnamic acid (12.0mmol) in 50ml dichloromethane in a 250ml round-bottomed reaction flask, and place under nitrogen protection at room temperature EDCI.HCl (18.0 mmol), DMAP (1.0 mmol) were added sequentially. After the addition is complete, heat-preserve and stir the reaction at 35° C., and post-process after detecting that the remaining amount of INB is less than 8% by HPLC. The reaction liquid was washed with 100 ml of purified water and 100 ml of 10% sodium chloride solution in turn, concentrated under reduced pressure and then used for later use.
[0107] b: Dissolve the above product in 50 ml of absolute ethanol in a 250 ml round-bottomed reaction bottle, and slowly add 5.0 mL of concentrated hydrochloric acid dropwise with stirring at room temperature. After the dropwise addition, stir and react at 80°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com