A kind of fluorinated camptothecin drug derivative and its preparation and application
A technology for fluorinated camptothecin and its derivatives, which is applied in the field of fluorinated camptothecin drug derivatives and their preparation and application, and can solve the problem of reducing the progress of research on the modification of phenolic compounds, many method steps, and low reaction yields, etc. problems, to achieve the effect of mild reaction conditions, simple operation process and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
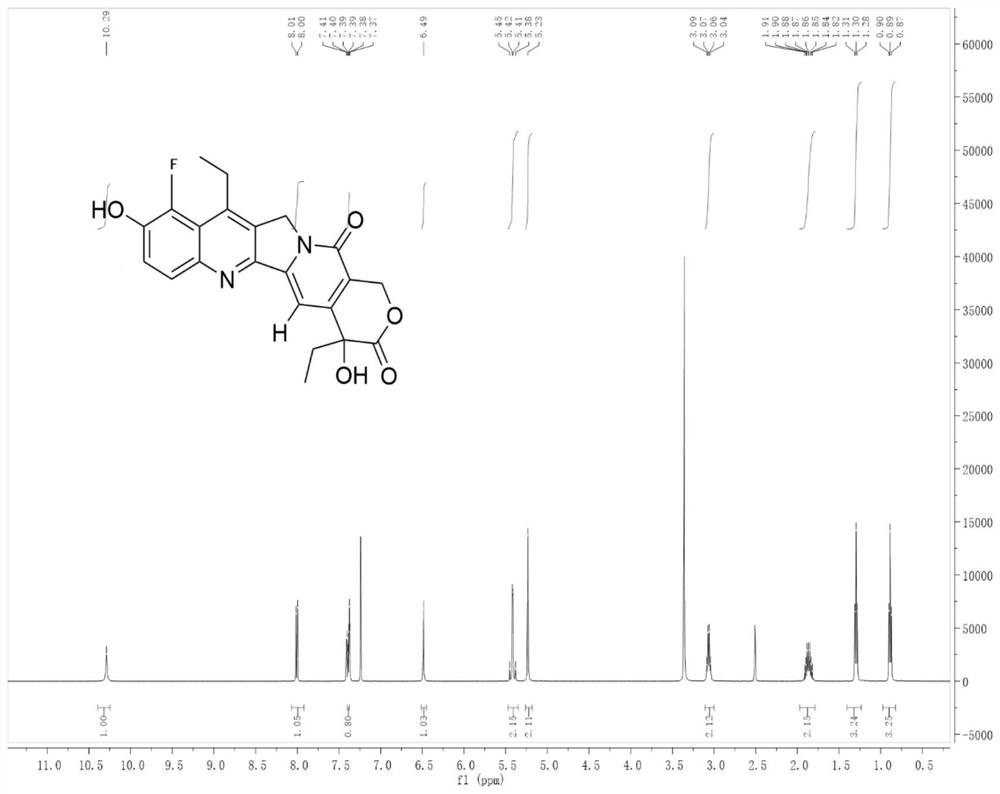
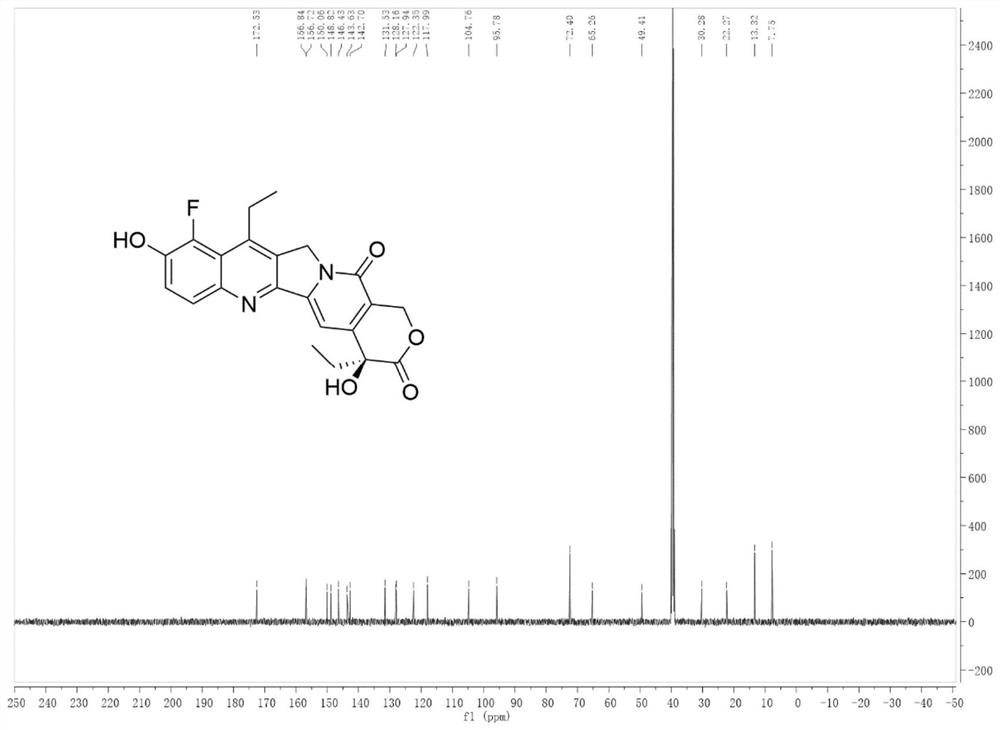
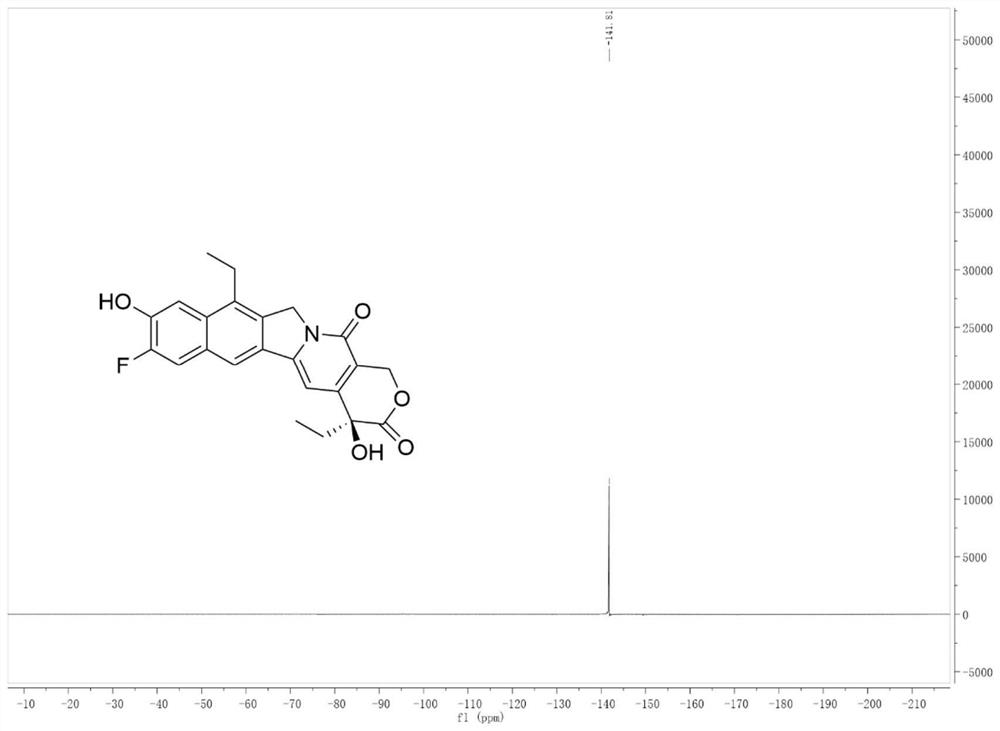
[0030] Embodiment 1: the preparation of compound (I)
[0031]
[0032] Add 1 mmol of camptothecin derivative SN-38 to 4 ml of 10% (w / w) acetic acid aqueous solution, add 1.5 mmol of Selectfluor, 0.05 mmol of Eosin Y, and react under 12W blue light irradiation at room temperature for 6 hours. After the reaction, the reaction liquid Saturated NaCl aqueous solution was added to the solution, extracted with dichloromethane, the organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain the crude compound. The crude compound was subjected to silica gel column chromatography, using a solution of ethyl acetate and petroleum ether with a volume ratio of 1:9 as the mobile phase, and the eluent with an Rf value of 0.3 to 0.5 was tracked and collected by TLC, and the collected eluent was passed through The solvent was removed under reduced pressure and dried to obtain 252 mg (yield 70%) of the pure compound shown in form...
Embodiment 2
[0034] Embodiment 2: Antitumor activity detection of compound (I)
[0035] The tumor cell Hela (cervical cancer cell) was selected, and the anti-proliferation activity of the tumor cell was detected by the MTT method. Cells were seeded at a concentration of 4000-5000 cells / well into a 96-well plate containing 10% fetal bovine serum in 1640 culture medium, and a note was added on the plate cover, and the cells were stored in 5% CO 2 , Cultivate at 37 DEG C for 12 hours, treat that the cells adhere to the wall on the 96-well plate, add the drug to be tested (compound (I) prepared in Example 1) with a pipette gun in the aseptic operating table, so that the drug concentration in each well is 0.01 μM, 0.1μM, 1μM, 10μM, 100μM five concentration gradients, each concentration set up five parallel groups, with anti-tumor drug SN-38 as a control), and put the 96-well plate in 5% CO 2 , and cultured at 37°C for 24 hours. Take out the 96-well plate, add 10 μL of MTT kit reagents (purcha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com