Method for establishing gastric cancer risk prediction model for gastric cancer diagnosis
A technology of risk prediction and gastric cancer model, which is applied in the field of establishing a risk prediction model of gastric cancer, can solve the problems of human incision trauma, difficulty in early diagnosis of gastric cancer, and low survival rate, and achieve the effect of improving early and accurate prediction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
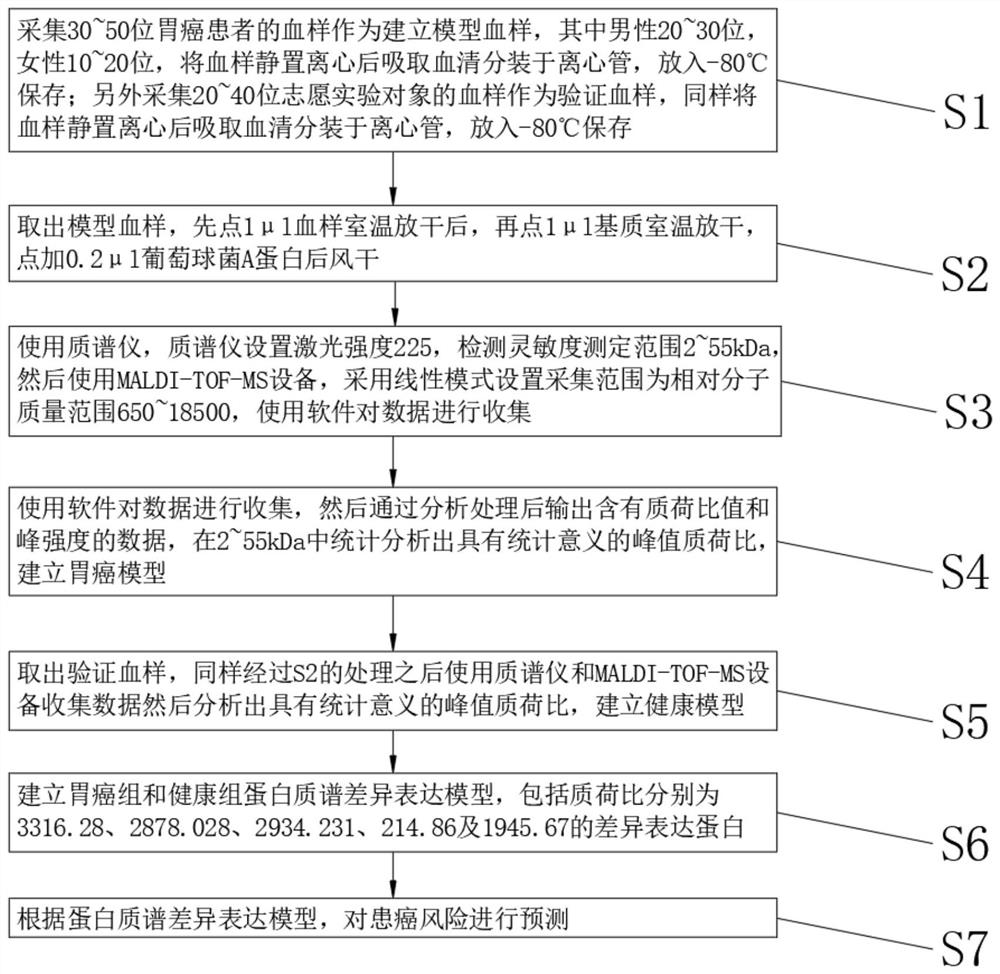
[0024] see figure 1 , the present invention provides a technical solution: a method for establishing a risk prediction model of gastric cancer for the diagnosis of gastric cancer, comprising the following steps:
[0025] S1. Collect blood samples from 30 voluntary patients with gastric cancer as model blood samples, including 20 males and 10 females. After the blood samples are left to stand and centrifuged, the serum is drawn and packed into centrifuge tubes, and stored at -80°C; another 20 volunteers are collected The blood sample of the experimental subject was used as the verification blood sample, and the blood sample was also left to stand and centrifuged, and the serum was collected and divided into centrifuge tubes, and stored at -80°C;
[0026] S2. Take out the model blood sample, first place 1 μl of blood sample and let it dry at room temperature, then place 1 μl of matrix and let it dry at room temperature, add 0.2 μl of Staphylococcus protein A and air dry;
[002...
Embodiment 2
[0040] see figure 1 , the present invention provides a technical solution: a method for establishing a risk prediction model of gastric cancer for the diagnosis of gastric cancer, comprising the following steps:
[0041] S1. Collect blood samples from 50 voluntary patients with gastric cancer as model blood samples, including 30 males and 20 females. After the blood samples are left to stand and centrifuged, the serum is drawn and packed into centrifuge tubes, and stored at -80°C; another 30 volunteers are collected The blood sample of the experimental subject was used as the verification blood sample, and the blood sample was also left to stand and centrifuged, and the serum was collected and divided into centrifuge tubes, and stored at -80°C;
[0042] S2. Take out the model blood sample, first place 1 μl of blood sample and let it dry at room temperature, then place 1 μl of matrix and let it dry at room temperature, add 0.2 μl of Staphylococcus protein A and air dry;
[004...
Embodiment 3
[0056] see figure 1 , the present invention provides a technical solution: a method for establishing a risk prediction model of gastric cancer for the diagnosis of gastric cancer, comprising the following steps:
[0057] S1. Collect blood samples from 40 voluntary patients with gastric cancer as model blood samples, including 25 males and 15 females. After the blood samples are left to stand and centrifuged, the serum is drawn and packed into centrifuge tubes, and stored at -80°C; another 40 volunteers are collected The blood sample of the experimental subject was used as the verification blood sample, and the blood sample was also left to stand and centrifuged, and the serum was collected and divided into centrifuge tubes, and stored at -80°C;
[0058] S2. Take out the model blood sample, first place 1 μl of blood sample and let it dry at room temperature, then place 1 μl of matrix and let it dry at room temperature, add 0.2 μl of Staphylococcus protein A and air dry;
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com