Progestogen formulations and uses thereof
A solvent and solubilizer technology, applied in the field of medicine, can solve pain and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-17
[0176] Example 1-17-Pharmacological study of HPC in smoke exposure-induced COPD rats
[0177] In order to examine the in vitro and in vivo efficacy of 17-HPC inhalation in chronic obstructive pulmonary disease (COPD) rats, a COPD rat model was established by exposing Wistar rats to CS for 1 hour per day for a total of 180 days, administered by aerosol inhalation. medicine. 72 Wistar rats were randomly divided into blank control group, model group, 17-HPC (0.5mg / ml, 0.25mg / ml and 0.1mg / ml) group, BUD (0.5mg / ml and 0.1mg / ml) group , and combined treatment (0.5mg / ml BUD+0.25mg / ml 17-HPC and 0.1mg / ml BUD+0.25mg / ml 17-HPC) group. The drug concentration is the concentration of the nebulized solution. Rat lung function (forced vital capacity) was measured using a small animal spirometer and differential counts in the BALF were determined microscopically. Serum and BALF cytokine (IL-6, IL-17, TNF-α and IL-β) levels were measured by ELISA. HE staining was used to measure the infilt...
Embodiment 2
[0183] Example 2-Seven-day atomization inhalation toxicity study on rats
[0184] A 7-day inhalation toxicity study in rats was conducted under GLP conditions. In this study, Sprague-Dawley rats (10 / sex / group) were administered aerosolized 17-HPC at 8, 24 and 72 mg / kg / day for 7 consecutive days. Rats in the control group were given nebulized vehicle. Animals were observed daily for clinical signs. Body weight and food consumption were recorded at pretest and then on days 1, 4 and 7. To assess the reversibility of 17-HPC treatment, groups of satellite rats (5 / sex / group) were dosed by inhalation at 72 mg / kg / day for 7 days and sacrificed on day 14. All animals were sacrificed on day 8 except rats in the satellite group. Hematological and biochemical tests were performed at the end of the study. Organ weights, gross pathology and histopathology assessments were performed.
[0185] The preliminary results of this experiment showed that no clinical symptoms and body weight cha...
Embodiment 3
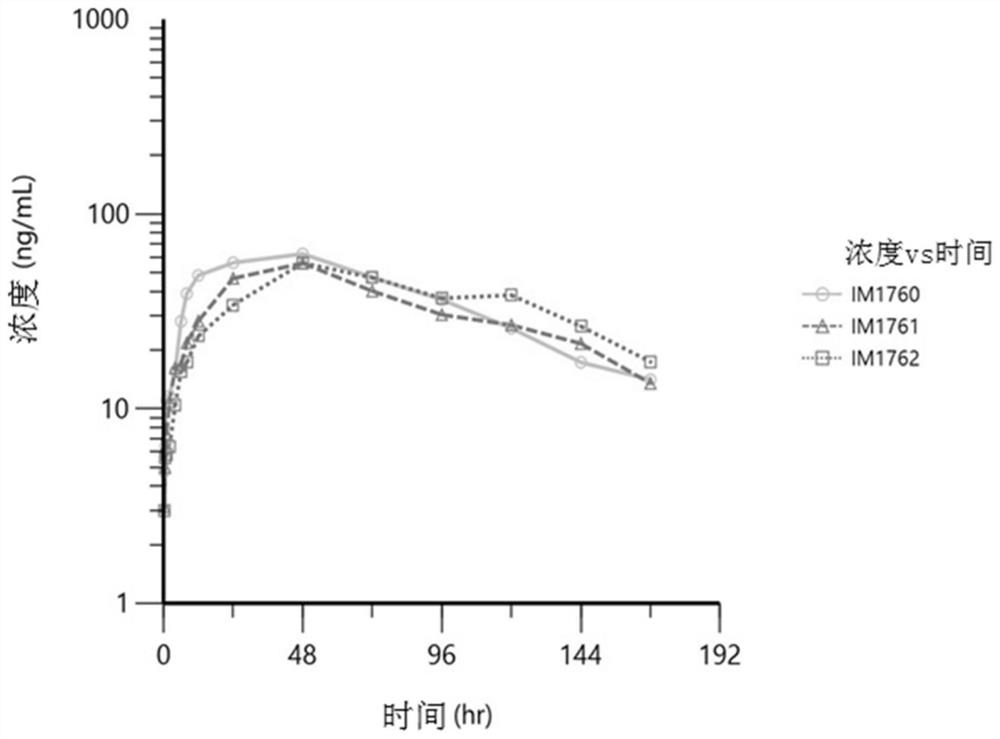
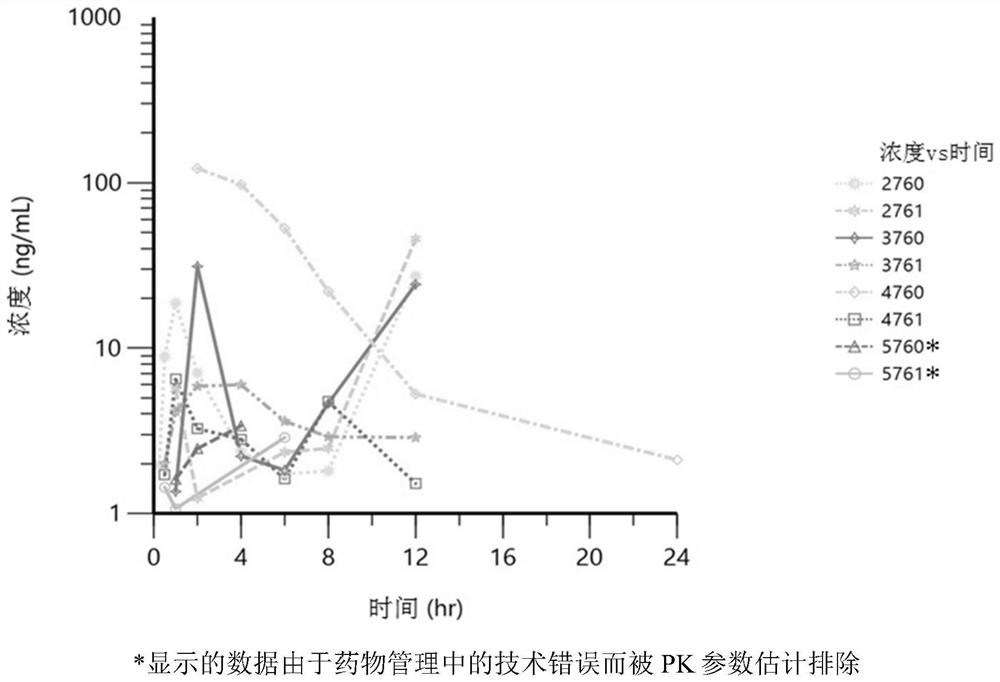
[0187] Example 3-PK Analysis Report of Oral PR2005 Formula for Bitches
[0188] In this study, the pharmacokinetic parameters of the experimental drug PR2005 in different formulations were determined after oral administration in dogs. The study further determined the relative bioavailability of the test drug PR2005 in each formulation, compared to the intramuscular solution.
[0189] PK analysis method:
[0190] Estimation of PK parameters, including maximum plasma concentration (Cmax), time to Cmax (Tmax), time lag during absorption (Tlag), plasma concentration-time profile from time 0 to the last quantifiable data point (AUClast) Area under (AUCinf), plasma half-life (t1 / 2), apparent plasma clearance (CL / F), apparent volume of distribution (Vz / F). All these parameters were calculated using standard non-isolated analysis methods. Geometric means and %CVs are reported for most PK parameters, and median and ranges for Tmax and Tlag are reported. Plasma PR2005 PK data of Phe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap