Application of a kind of hoip inhibitor for preparing medicine for treating type II human telangiectasia
A telangiectasia drug technology, applied in the preparation of drugs for the treatment of type II human telangiectasia, in the field of HOIP inhibitors, can solve problems that have not been reported in the research, and achieve the effect of enhancing the level of linear ubiquitination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
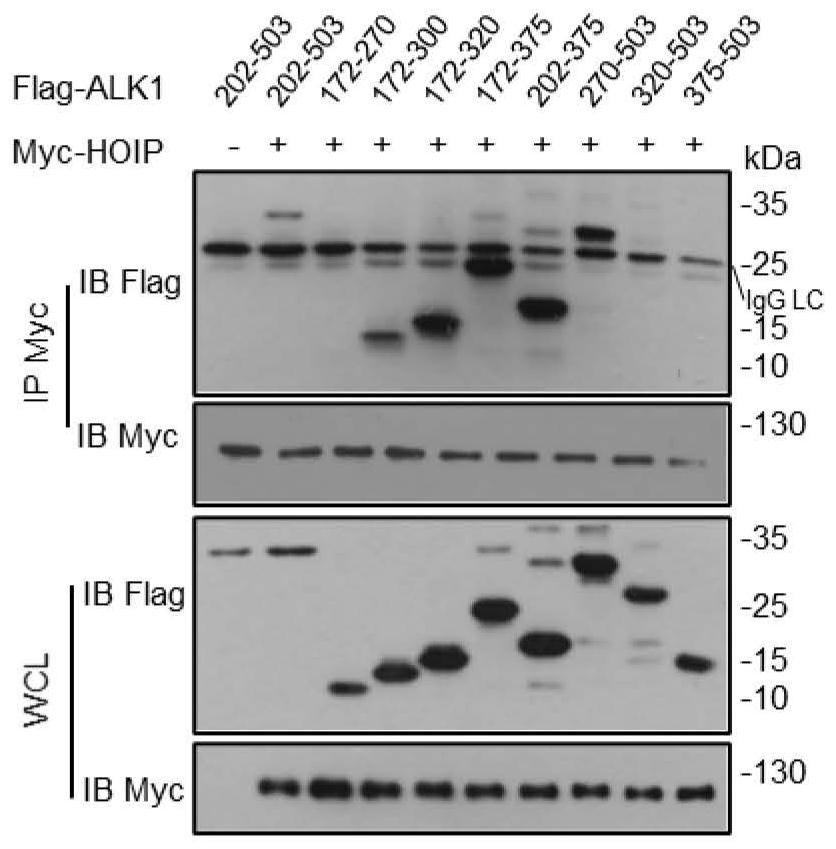
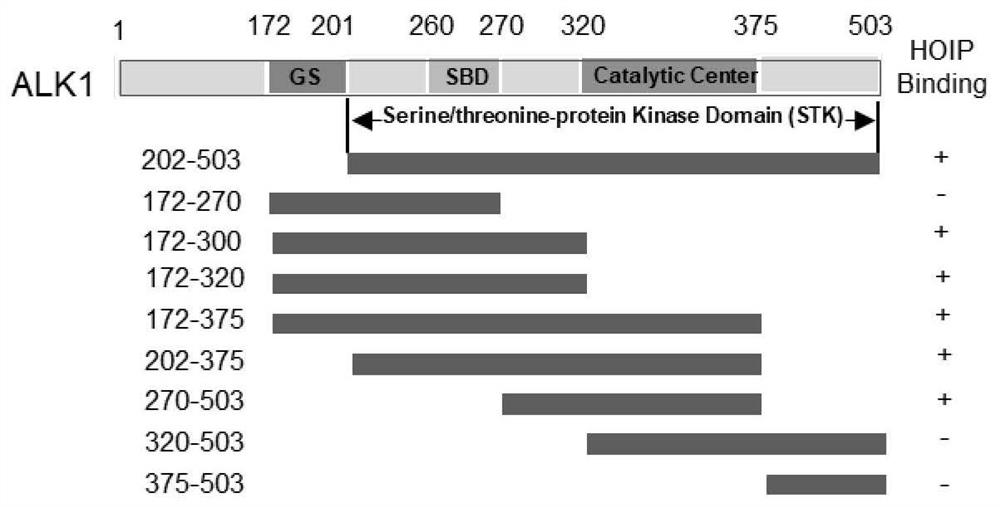
[0044] Example 1 demonstrates that ALK1 270-320aa (amino acids) domain specifically binds to HOIP
[0045] In order to confirm the specific domain of ALK1 binding to HOIP, HEK293T purchased from ATCC was preferred to overexpress the Flag-ALK1 series truncated body ( figure 2 ) and Myc-HOIP plasmid, and the interaction between the two was detected by immunoprecipitation technique.
[0046] The protein co-immunoprecipitation steps are:
[0047] 1. Collect the cells that have been transfected with the plasmid for 48 hours (you can choose culture flask or 6-well plate for transfection);
[0048] 2. Wash 3 times with pre-cooled PBS, centrifuge at 2000rpm for 3min;
[0049] 3. Add 500 μl of lysate (choose HEPES lysate or RIPA lysate according to specific experimental requirements), then choose to lyse on ice for 30 minutes or directly sonicate the cells, and then centrifuge at 12,000 rpm for 10 minutes to obtain the supernatant for later use;
[0050] 4. Take 50 μl of the supern...
Embodiment 2
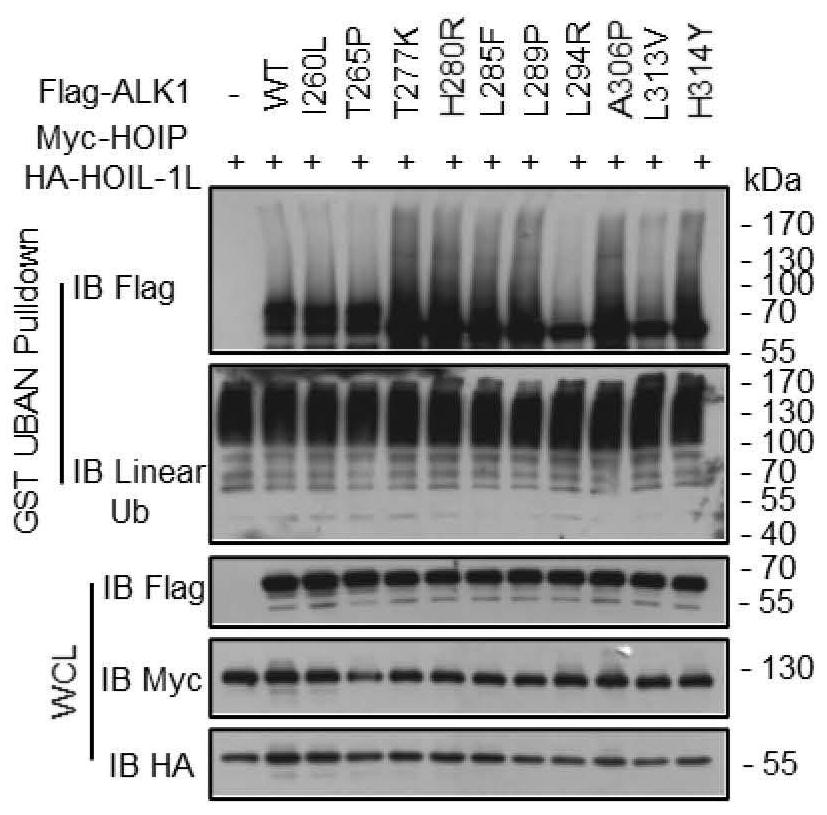
[0068] Example 2 demonstrates the relationship between HHT2-related mutations and linear ubiquitination
[0069] To further explore the physiological significance of HOIP binding to ALK1, we combined the existing UniProt (https: / / www.uniprot.org / uniprot / P37023) database to screen 8 HHT2-related mutations (T277K, H280R, L285F , L289P, L294R, A306P, L313V, H314Y), using the same method as in Example 1 to overexpress the Flag-ALK1 series mutants and Myc-HOIP plasmids in HEK293T to detect the linear ubiquitination level ( image 3 ), six of the eight mutations (T277K, H280R, L285F, L289P, A306P, H314Y) were found to have significantly enhanced linear ubiquitination levels relative to wild-type ALK1 ( image 3 and Figure 4 ).
[0070] At the same time, it was found that the binding ability of these six mutants to HOIP was significantly enhanced by immunoprecipitation technique ( Figure 5 ). The ubiquitination experiment operation is as follows:
[0071] 1. Collect the cells ...
Embodiment 3
[0077] Example 3 Linear ubiquitination modification inhibits the kinase activity of ALK1
[0078] In order to verify the effect of these mutations on enhancing the ability to bind HOIP and increasing the ubiquitination level, we need to first figure out how the ubiquitination modification affects ALK1 itself. ALK1 is a classic serine-threonine kinase, and its kinase activity is the key to its function. The common detection of ALK1 kinase activity is to detect by constructing and purifying the constitutive activator of ALK1. Therefore, we found that the constitutive activator of ALK1 can phosphorylate the substrate Smad1 through in vitro phosphorylation experiments, but the constitutive activator modified by linear ubiquitination lost its activity. Therefore, the activity of ALK1 kinase after enhanced ubiquitination of mutants was proved by in vitro phosphorylation experiments. The in vitro phosphorylation experiment steps are as follows:
[0079] 1. HEK293T cells were transf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com