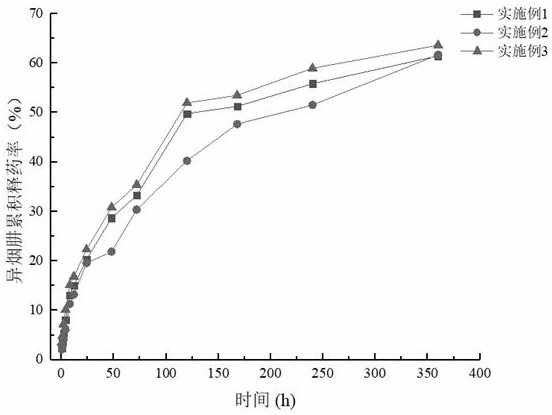
Isoniazide and rifampicin co-loaded microsphere sustained release preparation and preparation method thereof
A slow-release preparation and isoniazid technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of organic solvent residues, easy adhesion and aggregation of particles, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0031] Experimental example 1 In vitro analysis method experiment of isoniazid and rifampicin
[0032] Rifampicin and isoniazid UV spectrophotometry: After full-wavelength scanning, rifampicin has maximum absorption at 238 nm, 254 nm and 334 nm wavelength, and minimum absorption at 298 nm wavelength. Isoniazid has an absorption maximum at a wavelength of 262 nm. In the comprehensive analysis, 262 nm was used as the detection wavelength of rifampicin and isoniazid analysis methods.
[0033]HPLC detection methodology for rifampicin and isoniazid: Chromatographic column: Besil-C18 (5 μm, 4.6×250 mm), UV spectrophotometer detection wavelength: 262 nm, injection volume: 20 µL, column temperature: 25°C , flow rate: 1.0 mL / min, mobile phase A: disodium hydrogen phosphate solution (0.02 mol / L) and adjust the pH value to 4.0 with phosphoric acid, mobile phase B: methanol: acetonitrile (volume ratio 1:2). The volume ratio of mobile phase A and B was kept constant at 95:5 in the first ...
experiment example 2
[0034] Experimental example 2 Prescription optimization of PLGA microsphere preparation process
[0035] 1. Single factor investigation: According to the preparation method, select the ratio of phospholipids and cholesterol, the amount of phospholipids and cholesterol added, the rotational speed, the concentration of PVA in the water phase, the concentration of PLGA in the oil phase, the volume ratio of oil to water, voltage, receiving distance, injection speed, dosage, dosage The proportion and the proportion of dichloromethane and absolute ethanol in the oil phase are used as the influencing factors of microsphere quality control, and the single factor experiment is carried out with the average particle size and encapsulation efficiency as evaluation indicators. From the experimental results, it can be seen that the addition of phospholipid cholesterol is between 1.4% and 5.6%, the oil-water volume ratio is between 1:8 and 1:10, the receiving distance is between 10 and 15 cm,...
Embodiment 1
[0044] Co-loaded isoniazid and rifampicin lung targeting PLGA microsphere preparation, the preparation method is:
[0045] (1) The water phase is precisely weighed 26 mg of isoniazid and added to 0.5 mL of 1.4% PVA aqueous solution, and bathed in water at 40°C for 10 minutes;
[0046] (2) The oil phase is a mixed solution of precisely weighed rifampicin 26 mg, 7% PLGA (lactic acid-glycolic acid 75:25), phospholipid 60 mg and cholesterol 10 mg dissolved in 5 mL of dichloromethane and absolute ethanol , ultrasonication in a water bath for 5 min facilitates its dissolution;
[0047] (3) Add the water phase to the oil phase, mix, homogenize, and emulsify for 2 minutes with a hand-held homogenizer at 1000 rpm, and then draw it into a 5mL syringe;
[0048] (4) Place the syringe on the injection pump of the electrospinning machine, and choose a needle of 21 # , to establish a high-voltage electric field between the needle and the receiving device. After setting the voltage of 8 kV...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com