Picane maleate agar liposome and preparation method thereof
A technology of picentan maleate and liposome, applied in the field of medicine, can solve the problems of inability to prepare samples, low encapsulation efficiency, drug leakage, etc., and achieve excellent anti-tumor effect, reduce toxic reactions, and be safe and reliable. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
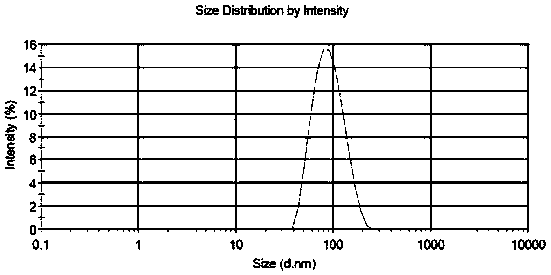
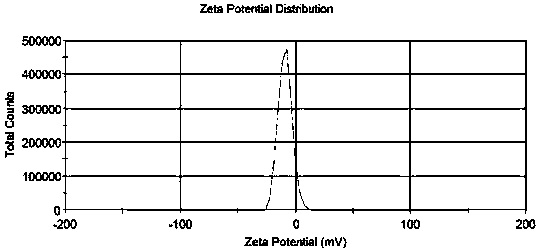
[0030] The encapsulation efficiency of the liposomes prepared by the different methods of Examples 1-9 of the present invention is shown in Table 1. In Example 5, dialysis was used to remove the ammonium sulfate solution in the external aqueous phase. Although the encapsulation efficiency reached 89.6%, it was not suitable for industrial production. In Examples 7 and 8, a buffer was used to adjust the pH value, and the liposome encapsulation efficiency was only 4%. Embodiment 9 adopts high-pressure homogenizer-extruder to prepare the liposome particle diameter of Picetaxel Maleate to be 87.29nm, uniform particle size distribution, distribution width 40.15, potential-9.06mV, the results are shown in figure 1 and figure 2 .
[0031] The encapsulation efficiency of the Picetaxel maleate liposome prepared in the embodiment of table 1
[0032]
Embodiment 1
[0034] (1) Film formation: Weigh 300.2mg of DSPC, 100.2mg of CHO, 99.8mg of DSPE-PEG20000, dissolve in 10mL of chloroform, heat to dissolve, remove the chloroform by evaporating under reduced pressure on a rotary evaporator at 55°C, and obtain lipid film;
[0035] (2) Hydration: add 26mL of 0.2mol / L ammonium sulfate solution to the lipid film, and hydrate at 55°C for 30min to obtain the primary blank liposome;
[0036] (3) Sizing: Ultrasonic disperse the blank liposome primary product on an ultrasonic disperser for 4 minutes with a power of 600-800w. After sizing, filter with a 0.45-micron microporous membrane for 3 times and a 0.22-micron microporous membrane for 1 time. , to obtain blank liposome 24mL;
[0037] (4) Manufacturing liposome transmembrane gradient inside and outside: adopt ultrafiltration method, replace the ammonium sulfate solution of blank liposome external water phase with 9% sucrose solution, replace 7 times, obtain blank liposome 20mL;
[0038] (5) Drug ...
Embodiment 2
[0041] (1) Oil phase: Weigh 450.6mg of DSPC, 150.3mg of CHO, and 149.9mg of DSPE-PEG2000, dissolve in 3.9mL of absolute ethanol, heat to dissolve, and keep warm at 55°C for 30min;
[0042] (2) Water phase: prepare 39 mL of 0.2 mol / L ammonium sulfate aqueous solution, and keep it warm at 55 °C for 30 min;
[0043] (3) Emulsification: inject the oil phase into the water phase at 55°C, and stir continuously with a magnetic stirrer until the absolute ethanol is completely volatilized to obtain 37 mL of blank liposome;
[0044](4) Sizing: Ultrasonic disperse the blank liposome primary product on an ultrasonic disperser for 4 minutes with a power of 600-800w. Filter once to obtain 35 mL of blank liposomes;
[0045] (5) Manufacturing liposome transmembrane gradient inside and outside: adopt ultrafiltration method, replace the ammonium sulfate solution of blank liposome external water phase with 9% sucrose solution, ultrafilter 7 times, obtain blank liposome 30.8mL;
[0046] (6) Dru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com