Maleic acid pixantrone liposomal and preparing method thereof
A technology of picentan maleate and liposome, applied in the field of medicine, can solve the problems of inability to prepare samples, low encapsulation rate, drug leakage, etc., and achieves a solution with reduced toxicity, excellent anti-tumor effect, and reliable safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
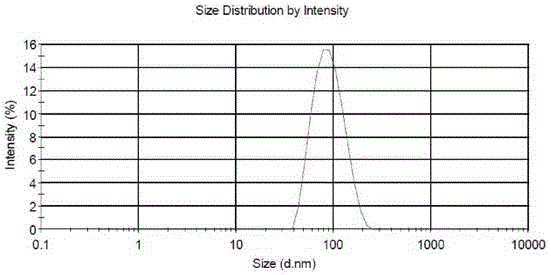
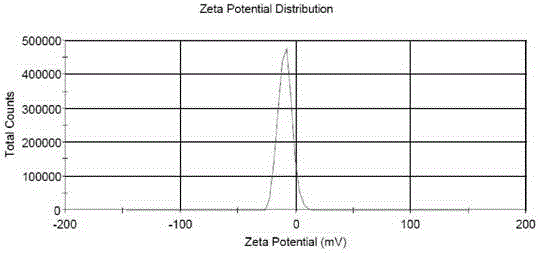
[0030] The encapsulation efficiency of the pizent agar liposomes prepared by the different methods of Examples 1-9 of the present invention is shown in Table 1. In Example 5, the dialysis method was used to remove the ammonium sulfate solution in the outer water phase. Although the encapsulation rate reached 89.6%, it was not suitable for industrial production. In Examples 7 and 8, the pH value was adjusted with buffer, and the liposome encapsulation efficiency was only 4%. Embodiment 9 adopts the high pressure homogenizer-extruder to prepare the particle size of picanthane maleate liposome is 87.29nm, the particle size distribution is uniform, the distribution width is 40.15, the potential-9.06mV, the result sees figure 1 and figure 2 .
[0031] The encapsulation efficiency of the pizent agar liposome prepared in the embodiment of table 1
[0032]
Embodiment 1
[0034] (1) Film formation: Weigh DSPC300.2mg, CHO100.2mg, DSPE-PEG200099.8mg, dissolve in 10mL chloroform, heat to dissolve, evaporate under reduced pressure at 55°C on a rotary evaporator to remove chloroform to obtain lipids film;
[0035] (2) Hydration: add 26 mL of 0.2 mol / L ammonium sulfate solution to the lipid film, and hydrate at 55°C for 30 min to obtain a blank liposome first product;
[0036] (3) Granulation: ultrasonically disperse the blank liposomes on an ultrasonic disperser for 4 minutes, power 600-800w, filter 3 times with a 0.45-micron microporous membrane after granulation, and filter once with a 0.22-micron microporous membrane , to obtain 24 mL of blank liposomes;
[0037] (4) Making a transmembrane gradient inside and outside the liposome: using an ultrafiltration method, replace the ammonium sulfate solution in the outside water phase of the blank liposome with a 9% sucrose solution, and replace it 7 times to obtain 20 mL of the blank liposome;
[0038...
Embodiment 2
[0041] (1) Oil phase: Weigh DSPC450.6mg, CHO150.3mg, DSPE-PEG2000149.9mg, dissolve in 3.9mL absolute ethanol, heat to dissolve and keep at 55°C for 30min;
[0042] (2) Aqueous phase: prepare 39 mL of 0.2 mol / L ammonium sulfate aqueous solution, and keep it at 55°C for 30 min;
[0043] (3) Emulsification: inject the oil phase into the water phase at 55°C, and continuously stir with a magnetic stirrer until the absolute ethanol is completely volatilized to obtain 37 mL of blank liposomes;
[0044](4) Granulation: ultrasonically disperse the first blank liposomes on an ultrasonic disperser for 4 minutes, with a power of 600-800w, and filter 3 times with a 0.45-micron microporous membrane and a 0.22-micron microporous membrane after granulation. Filter once to obtain 35 mL of blank liposomes;
[0045] (5) Manufacture of liposome transmembrane gradient: adopt ultrafiltration method, replace the ammonium sulfate solution in the outer water phase of blank liposome with 9% sucrose so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com