Indole derivative and application thereof
A drug and compound technology, applied in the field of depression and anxiety therapeutic agents, compounds containing indole structure, can solve the problems of slow onset time, low cure rate, increased suicidal tendency, etc., and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
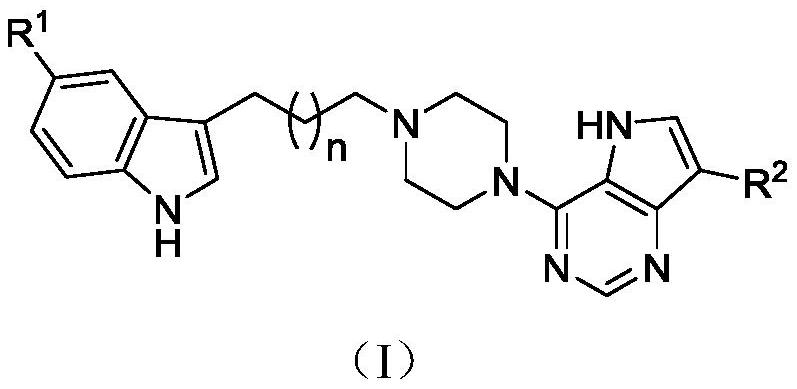
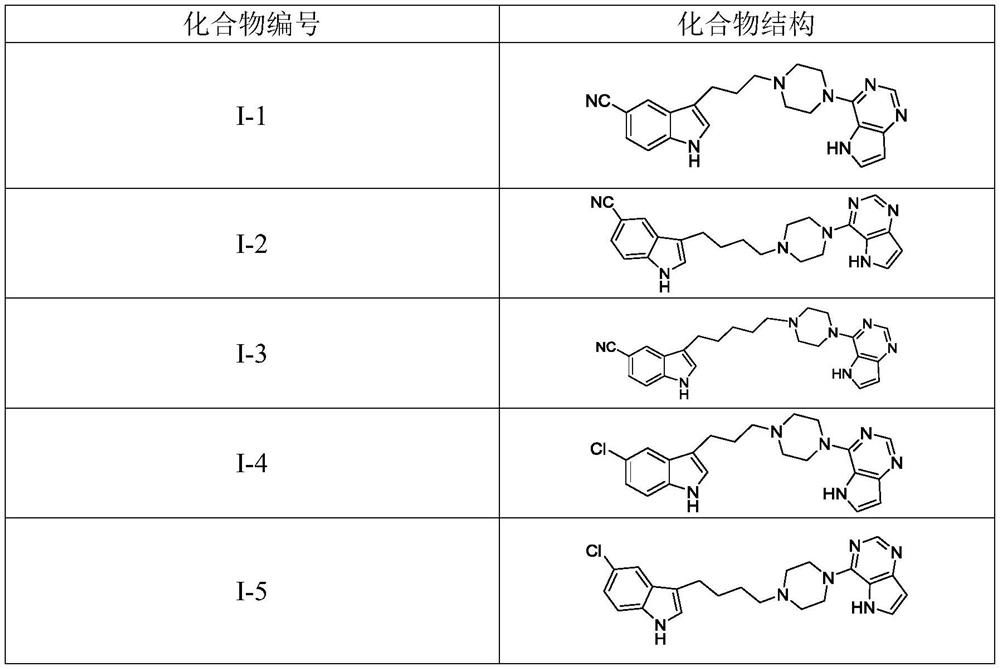
[0028] 3-(3-(4-(5H-pyrrolo[3,2-d]pyrimidin-4-yl)piperazin-1-yl)propyl)-5-cyano-1H-indole (I-1 )Synthesis
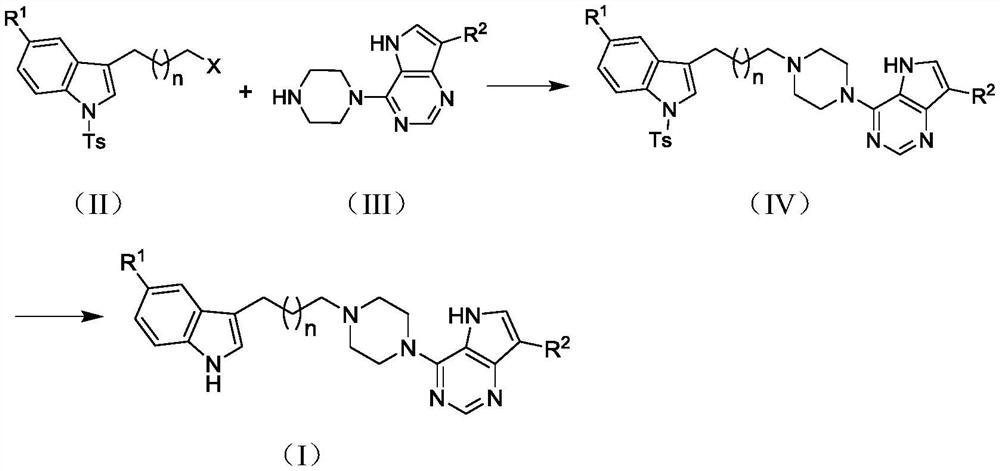
[0029] 1-p-Toluenesulfonyl-3-(3-(4-(5H-pyrrolo[3,2-d]pyrimidin-4-yl)piperazin-1-yl)propyl)-5-cyanindole Synthesis of (IV-1)
[0030] Using II-1 (1.0g, 2.7mmol) and 4-piperazinyl-5H-pyrrolo[3,2-d]pyrimidine hydrochloride (III-1, 0.96g, 4.0mmol) as raw materials, triethylamine (5ml), potassium iodide (appropriate amount), and DMF (50ml) were added to a 100ml eggplant-shaped bottle, added to an external temperature of 85°C and reacted for 12h before stopping. After the reaction solution was cooled, it was added to ice water (100ml), extracted with ethyl acetate (40ml×3), the organic layers were combined, washed with saturated brine (50ml×3), dried over anhydrous sodium sulfate, suction filtered, spin-dried, and columnar After separation by chromatography, 0.95 g of an off-white solid was obtained, with a yield of 65.65%.
[0031] 1 H-NMR (300MHz, CDCl 3 )δ(ppm): 1.80~1...
Embodiment 2
[0036] 3-(4-(4-(5H-pyrrolo[3,2-d]pyrimidin-4-yl)piperazin-1-yl)butyl)-5-cyano-1H-indole (I-2 )Synthesis
[0037] 1-p-Toluenesulfonyl-3-(4-(4-(5H-pyrrolo[3,2-d]pyrimidin-4-yl)piperazin-1-yl)butyl)-5-cyanindole Synthesis of (IV-2)
[0038] With II-2 (1.0g, 2.6mmol), III-1 (0.93g, 3.9mmol), triethylamine (5ml), potassium iodide (appropriate amount), DMF (50ml) as raw materials, the operation process is the same as compound VIII-1, 0.9 g of off-white solid was obtained with a yield of 62.82%.
[0039] 1H-NMR (300MHz, CDCl 3 )δ(ppm): 1.53~1.82(4H,m,-(CH 2 ) 2 CH 2 N3 ), 2.41 (2H, t, J=7.5Hz, ArCH 2 -), 2.51~2.64 (4H, m, piperazine hydrogen), 2.69 (2H, t, J=7.5Hz, -CH 2 N1 =8.6Hz,J 2 =1.5Hz, ArH),7.75(2H,d,J=8.4Hz,ArH),7.84(1H,s,ArH),8.05(1H,d,J=8.6Hz,ArH),8.47(1H,s, ArH).
[0040] 3-(4-(4-(5H-pyrrolo[3,2-d]pyrimidin-4-yl)piperazin-1-yl)butyl)-5-cyano-1H-indole (I-2 )Synthesis
[0041] Using IV-2 (1.0g, 1.81mmol), sodium hydroxide (1.1g, 27.2mmol) and methanol (50ml) a...
Embodiment 3
[0044] 3-(5-(4-(5H-pyrrolo[3,2-d]pyrimidin-4-yl)piperazin-1-yl)pentyl)-5-cyano-1H-indole (I-3 )Synthesis
[0045] 1-tosyl-3-(5-(4-(5H-pyrrolo[3,2-d]pyrimidin-4-yl)piperazin-1-yl)pentyl)-5-cyanindole ( IV-3) Synthesis
[0046] Using II-3 (1.0g, 2.25mmol) and III-1 (0.54g, 2.25mmol) as raw materials, the operation was the same as IV-1 to obtain 0.85g of off-white solid with a yield of 66.71%.
[0047] 1 H-NMR (300MHz, CDCl 3 )δ(ppm): 1.35~1.82(6H,m,-(CH 2 ) 3 CH 2 N3 ),2.40(2H,m,ArCH 2 -), 2.52~2.64 (4H, m, piperazine hydrogen), 2.68 (2H, t, J=7.5Hz, -CH 2 N<), 3.83~3.97 (4H, m, piperazine hydrogen), 6.59 (1H, d, J = 3.2Hz, ArH), 7.25 (2H, d, J = 8.4Hz, ArH), 7.35 (1H, d ,J=3.2Hz,ArH),7.44(1H,s,ArH),7.55(1H,d,J=8.6Hz,ArH),7.75(2H,d,J=8.4Hz,ArH),7.82(1H, s, ArH), 8.05 (1H, d, J=8.6Hz, ArH), 8.47 (1H, s, ArH).
[0048] 3-(5-(4-(5H-pyrrolo[3,2-d]pyrimidin-4-yl)piperazin-1-yl)pentyl)-5-cyano-1H-indole (I-3 )Synthesis
[0049] Add IV-3 (1.0g, 1.76mmol), sodium hydroxide ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap