Bi-functional humanized Anti-c5 antibodies and factor h fusion proteins and uses thereof
A fusion protein and antibody technology, applied in the direction of complement protein, hybrid immunoglobulin, animal/human protein, etc., can solve the problem of poor pharmacokinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
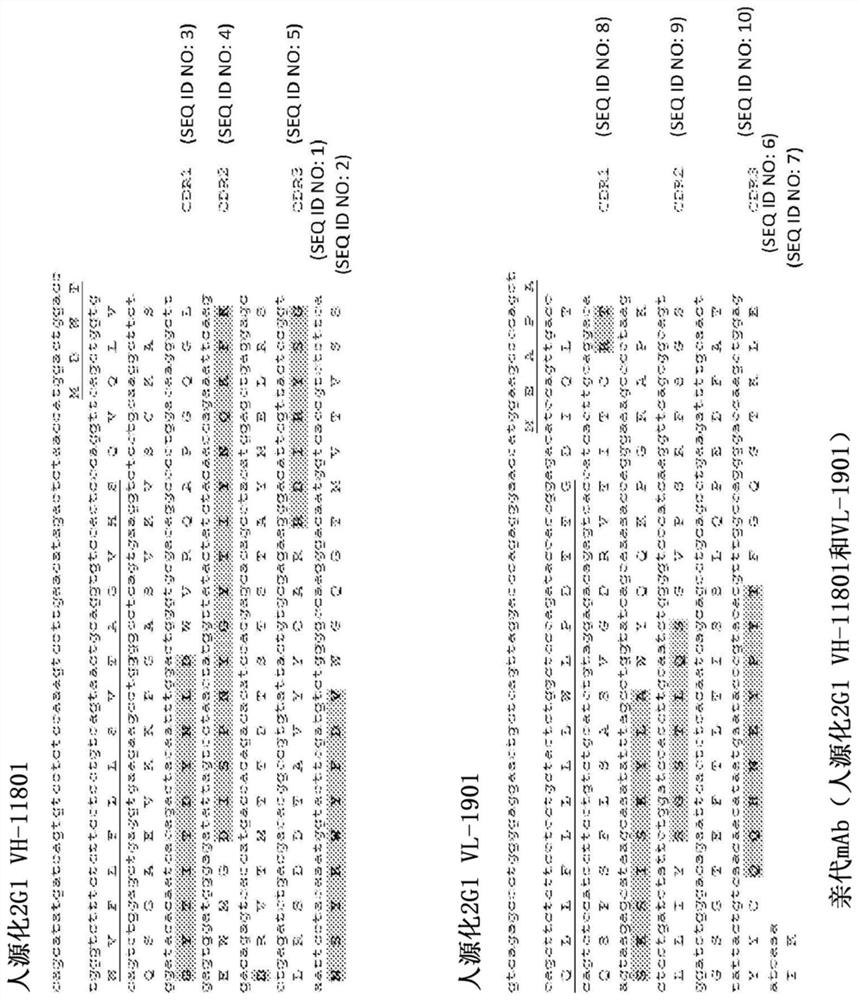
[0472] Variants of humanized 2G1 (VH-11801 (SEQ ID NO:2) and VL-1901 (SEQ ID NO:7)) were generated ( figure 1 ), with the aim of developing variants with improved binding to C5 at pH 7.4 and reduced binding to C5 at pH 5.8.
[0473] The methods described here are based on the understanding that the affinity and blocking potency of a mAb measured in vitro does not necessarily correlate with its in vivo half-life, PK or PD. This is at least in part because soluble antigens, such as C5, present in high concentrations in the blood form immune complexes (ie, mAbs that bind to the antigen) that are targeted for removal from the body. Therefore, in general, high antibody concentrations in the blood are required to block the in vivo activity of these soluble antigens.
[0474] The methods described herein are based on the understanding that the efficacy of therapeutic antibodies can be improved by increasing antibody recycling or half-life (and thus PK) and by accelerating the intrac...
Embodiment 2
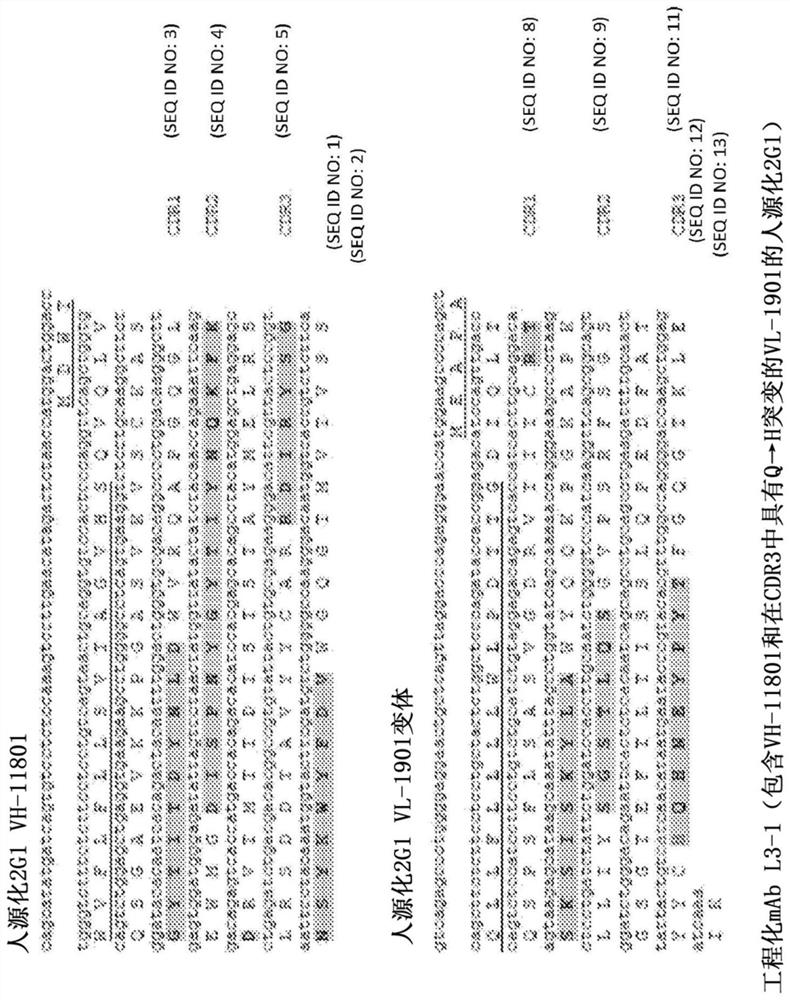
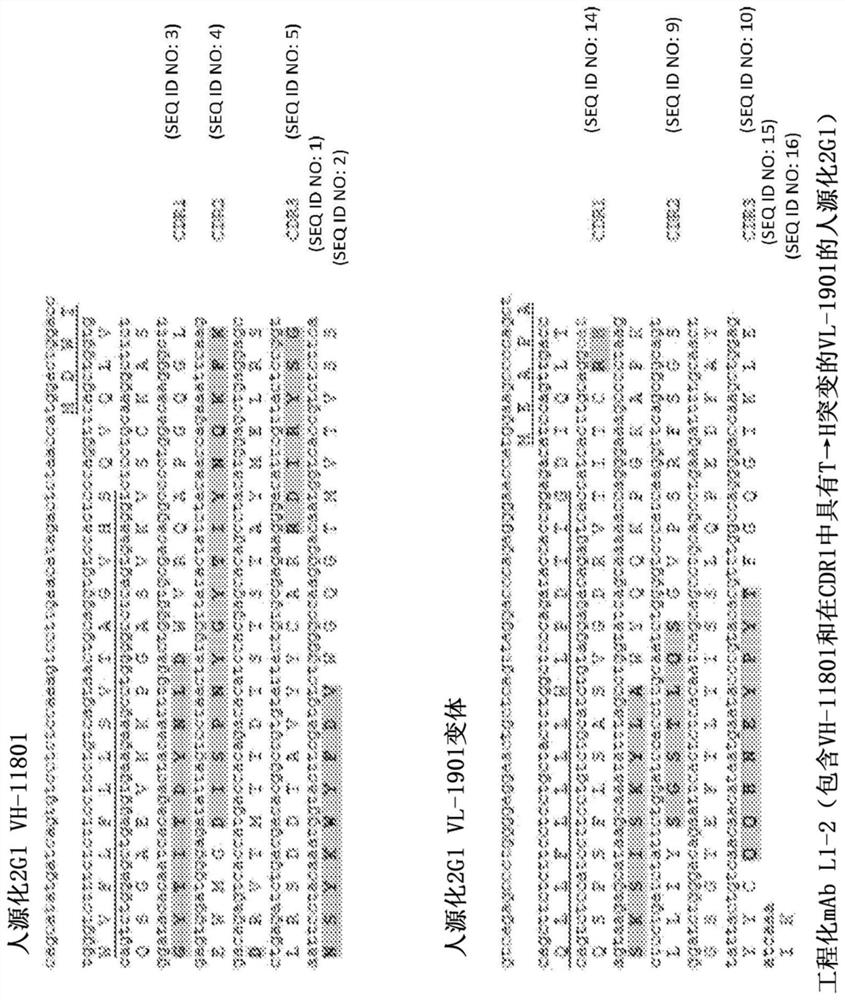
[0488] Based on the largest pH 7.4 vs pH 5.8 binding difference and the best pH 7.4 binding, variants IWW, IFW, FME and FMW were selected as the top 4 triple mutant clones of V1-8 / L1-9 for further characterization. performed additional analyzes and data mining from the affinity maturation project and identified the T-to-His mutation at position 9 of CDR2 as another promising random mutation that repairs pH 7.4 binding of V1-8 / L1-9 ( Figure 26 to Figure 28 ). IWW, IFW, FME and FMW were selected as the top 4 triple mutant clones of V1-8 / L1-9. IWW, IFW, FME and FMW were expressed and proteins were purified to confirm pH-dependent binding properties. C5 inhibitory activity was also tested in a hemolytic assay ( Figure 29 , Figure 30 , Figure 32 to Figure 34 ). The above 4 triple mutants were further combined with a new His mutation (CDR2 of VH, position 9, T to H mutation) to generate 4 quadruple mutants (IWWH, IFWH, FMEH and FMWH; for example, Figure 31 ). These new m...
Embodiment 3
[0492] Complement activation involves a cascade of target recognition and proteolytic cleavage. It can be activated by 3 different pathways, all of which converge at the C3 activation step ( Figure 43). These pathways are the classical, alternative and lectin pathways ( Figure 43 ). CP is activated by antigen-antibody complexes, and it includes subsequent C1 and C4 / C2 activation before the C3 activation step merges with other pathways. Once they bind to microbial surface sugar molecules, LPs are triggered by certain pattern recognition molecules such as MBL, collagen lectin and ficolin. It involves activation of MASP, which subsequently cleaves C4 / C2 and joins another pathway at the C3 activation step ( Figure 43 ). AP is constitutively active at low levels due to spontaneous hydrolysis and activation of C3 to generate C3(H2O). The latter can bind Factor B and, once activated by proteolysis of Factor D, generates the initial C3-cleaving enzyme complex C3(H2O)Bb( Fig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com