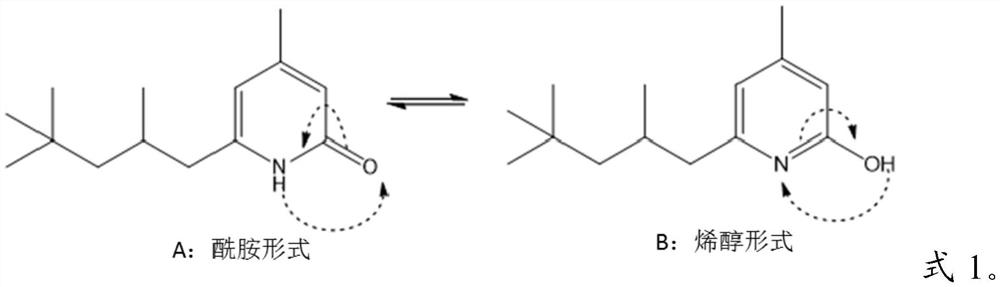
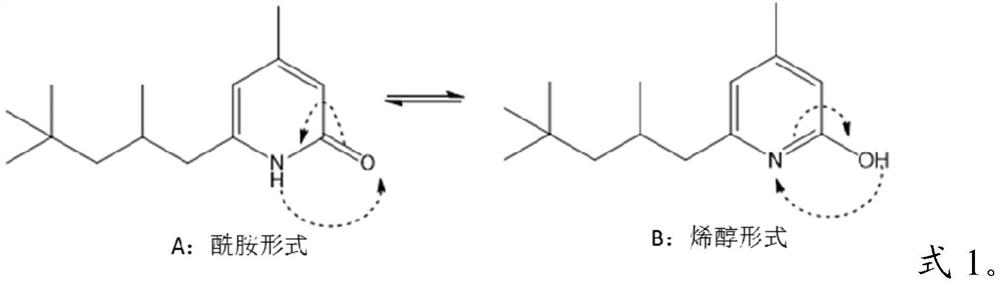
2(1H)-pyridinones and their use to treat inflammatory conditions
A technology of piroctone olamine salt and compound, which is applied in the field of new compound and its preparation, and can solve problems such as yellowing of color and light instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Preparation of compounds of formula 1
[0133] Compounds of formula 1 for experiments were prepared using the following materials, methods and processes:
[0134] UV processing
[0135] 0.05g in transparent glass vials Dissolved in 10 ml of methanol. exist UV irradiation in the MacBeth) Spectra Light III. UV mode selection UV illumination for both UVA and UVB light. The intensity of the light in this chamber is fixed (estimated UVA is 250 μW / cm 2 UVB is 110μW / cm 2 . The transmittance of UVA and UVB in the glass vial was 80.3% and 71.9%, respectively. The temperature of this chamber is equal to room temperature (20 ± 2 ° C). The sample is placed close to the center of the room. After 6 hours, the sample was concentrated by a rotary evaporator.
[0136] Separate
[0137] Separation was performed with a silica gel column (2 × 48 cm, v = 150 mL) filled with 60 g of silica gel (200-300). The silica gel column is activated with petroleum ether / ethyl acetate (2 / 1, v / v)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com