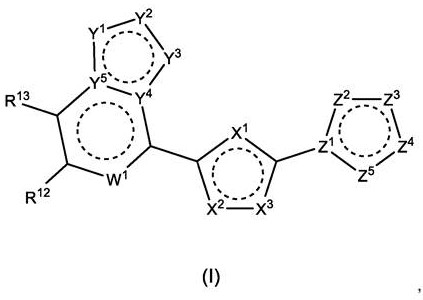
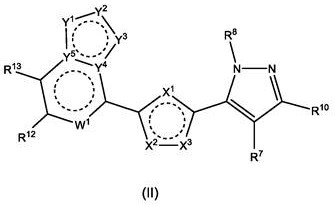
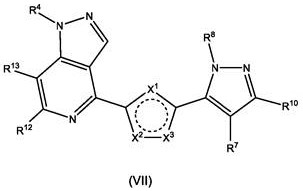
Polyheterocyclic modulators of STING (stimulators of interferon gene)
A technology of heterocyclic and heteroaromatic rings, applied in the field of polyheterocyclic regulators of STING (interferon gene stimulator)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment G01
[0867] Example G01: Preparation of 4-[4-(1-ethyl-3-methyl-1 according to Scheme G H -pyrazol-5-yl)-1 H -Imidazol-2-yl]-1-methyl-1 H -Indazole-6-carboxamide.
[0868] Scenario G:
[0869]
[0870] Step 1: Synthesis of 6-bromo-1-methyl-1H-indazole-4-carboxylic acid 2-(1-ethyl-3-methyl-1 H - Pyrazol-5-yl)-2-oxoethyl ester (G-1).
[0871] To 6-bromo-1-methyl-1 under nitrogen H -Indazole-4-carboxylic acid (Int-HG-13) (280 mg, 1.10 mmol) and 2-bromo-1-(1-ethyl-3-methyl-1 H To a suspension of -pyrazol-5-yl)ethan-1-one (Int-TG-07) (279 mg, 1.21 mmol) was added DIPEA (0.50 mL, 3.00 mmol). The yellow solution was stirred at 25°C for 17 hours. LCMS analysis indicated complete consumption of starting material. The solution was concentrated in vacuo to provide the title compound (G-1), which was used in the next step without further purification. 1 H NMR (400 MHz, methanol-d 4 ) δ = 8.48 (s, 1H), 8.18 (s, 1H), 8.05(d, J = 1.5 Hz, 1H), 6.98 (s, 1H), 5.57 (s, 2H), 4.50 (q, J ...
Embodiment H01
[0880] Example H01: Preparation of 4-[4-(1-ethyl-3-methyl-1 according to Scheme H H -Pyrazol-5-yl)-1,3-thiazol-2-yl]-1-methyl-1 H -Indazole-6-carboxamide.
[0881] Scenario H:
[0882]
[0883] Step 1: Synthesis of 6-bromo-4-[4-(1-ethyl-3-methyl-1 H -Pyrazol-5-yl)-1,3-thiazol-2-yl]-1-methyl-1 H -Indazole (H-1).
[0884] to 6-bromo-1-methyl-1 H -Indazole-4-carbothioamide (Int-HG-14) (93.5 mg, 0.346 mmol) in MeOH (3.0 mL) to a suspension of 2-bromo-1-(1-ethyl-3- methyl-1 H - Pyrazol-5-yl)ethan-1-one (Int-TG-07) (105 mg, 0.454 mmol). The reaction was heated at 80 °C for 14 h. LCMS analysis indicated consumption of starting material and formation of product mass. The reaction was concentrated in vacuo to provide a white solid. The solid was triturated with DCM (5.0 mL) and then dried under vacuum to afford the title compound (H-1) (65 mg, 47% yield) as a white solid. 1 HNMR (400 MHz, DMSO-d 6 ) δ = 8.57 (s, 1H), 8.22 (s, 1H), 8.19 (s, 1H), 7.87 (d, J = 1.3 Hz, 1H)...
Embodiment J01
[0889] Example J01: Preparation of 4-[3-(1-ethyl-3-methyl-1 according to Scheme J H -pyrazol-5-yl)-1-methyl-1 H -1,2,4-Triazol-5-yl]-1-methyl-1 H -Pyrazolo[4,3- c ]pyridine-6-carboxamide.
[0890] Scenario J:
[0891]
[0892] Step 1: Synthesis of 6-chloro-4-[3-(1-ethyl-3-methyl-1 H -pyrazol-5-yl)-1-methyl-1 H -1,2,4-Triazol-5-yl]-1-methyl-1 H -Pyrazolo[4,3- c ]pyridine (J-1)
[0893] to 3-(1-ethyl-3-methyl-1 H -pyrazol-5-yl)-1-methyl-1 H To a suspension of -1,2,4-triazole (Int-TG-04) (55.6 mg, 0.291 mmol) in toluene (1.5 mL) was added 4,6-dichloro-1-methyl-1 H -Pyrazolo[4,3- c ]pyridine (Int-HG-08) (88.7 mg, 0.439 mmol), Pd(OAc) 2 (6.5 mg, 0.029 mmol), P( n -Bu)Ad 2 (21.8 mg, 0.061 mmol), PivOH (8.9 mg, 0.087 mmol) and K 2 CO 3 (129.8 mg, 0.939 mmol). The solution was sparged with nitrogen for 2 minutes and then sealed. The reaction was heated at 120 °C for 16 h. LCMS analysis indicated that most of the starting triazole had been consumed and formatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com