Novel antimalarial drug
A compound, pyrazole technology, applied in the direction of anti-infective drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as damage to chemotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Example 1: Synthesis of compounds according to the invention
[0184] The compounds of the present invention can be prepared from readily available starting materials using methods and procedures known to those skilled in the art. It should be understood that where typical or preferred experimental conditions (ie, reaction temperatures, times, moles of reagents, solvents, etc.) are given, other experimental conditions may also be used unless otherwise indicated. Optimal reaction conditions may vary with the particular reactants or solvents used, but these conditions can be determined by one skilled in the art using routine optimization procedures.
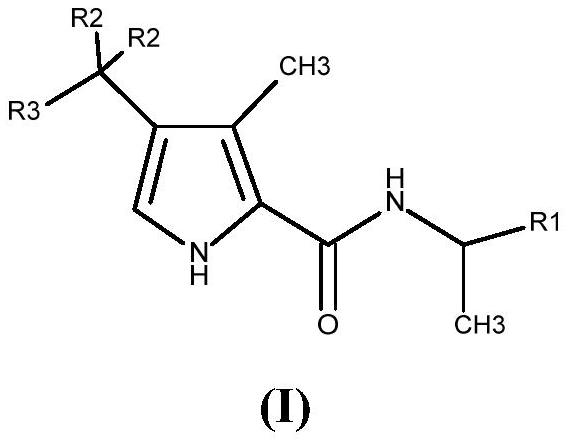
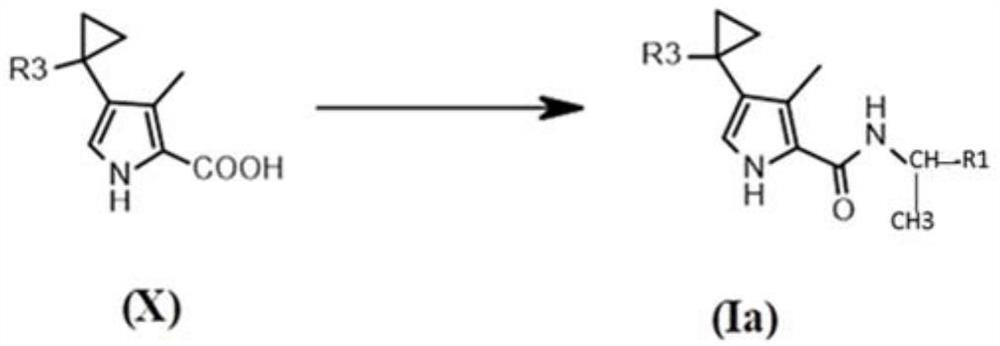
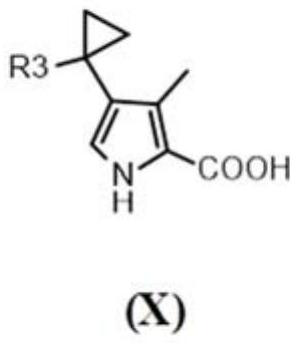
[0185] Compounds 1 to 36 of the present invention were synthesized as described in the general synthetic routes described herein. In particular, for compounds of formula (I), when two R 2 When linked to form an optionally substituted cyclopropyl, compounds were synthesized according to Scheme 3 below.
[0186] Scenario 3...
Embodiment 2
[0346] Example 2: Method for Assessing Solubility of Compounds of the Invention
[0347] The solubility and metabolic stability of the compounds of the present invention were assessed according to the protocol detailed in Phillips et al., 2015, supra, and are listed in Table 1 below, in comparison to DSM 265 having the following structure:
[0348]
[0349] Table 1
[0350]
[0351]
[0352] These data support the significantly improved solubility of the compounds of the present invention over DSM265. In addition, compound 14 is particularly stable in metabolism.
Embodiment 3
[0353] Example 3: The activity of the compound against Plasmodium and mammalian DHODH indicates that the compound is effective against Plasmodium enzymes selective activity.
[0354] Protein expression and purification. Contains His 6 BL21-DE3 E. coli labeled DHODH-pRSETb (N-terminal tag) (Pf and PvDHODH), pET22b C-terminal tag (human) or pET-28b C-terminal tag (rat, mouse and dog) constructs (E coli) phage-resistant cells were cultured and harvested with appropriate antibiotics, and proteins were purified using HisTrap HP columns, followed by gel filtration as previously described (Coteron et al., 2011, J.Med Chem), Vol. 54, No. 15, pp. 5540-5561; Phillips et al., 2015, supra; White et al., 2019, ACS Infect Dis, Vol. 5, No. 1 , pp. 90-101). The concentration of purified DHODH was determined based on FMN absorbance at 454 nm (ε445 = 12.5 mM -1 cm -1 ) (Malmquist et al., 2008, Biochemistry, Vol. 47, No. 8, pp. 2466-2475).
[0355] DHODH kinetic analysis. The 50% inhi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap