Anti-C5 antibodies for treatment of neuromyelitis optica lineage disorders
A technology for neuromyelitis optica and antibodies, which can be applied to nervous system diseases, antibodies, antibody medical components, etc., and can solve the problems of unmet medical needs in the effective and safe treatment of NMO
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0138] Example 1. Efficacy and safety of eculizumab in adult patients with neuromyelitis optica spectrum disorder (NMOSD)
[0139] 1. Research Design
[0140] 1.1. Overall Design
[0141] This is a Phase 3, external placebo-controlled, open-label, multicenter study to evaluate the efficacy and safety of eculizumab in adult patients with NMOSD. Approximately 55 eligible adult patients with NMOSD from North America, Europe, Asia Pacific and Japan participated in the study.
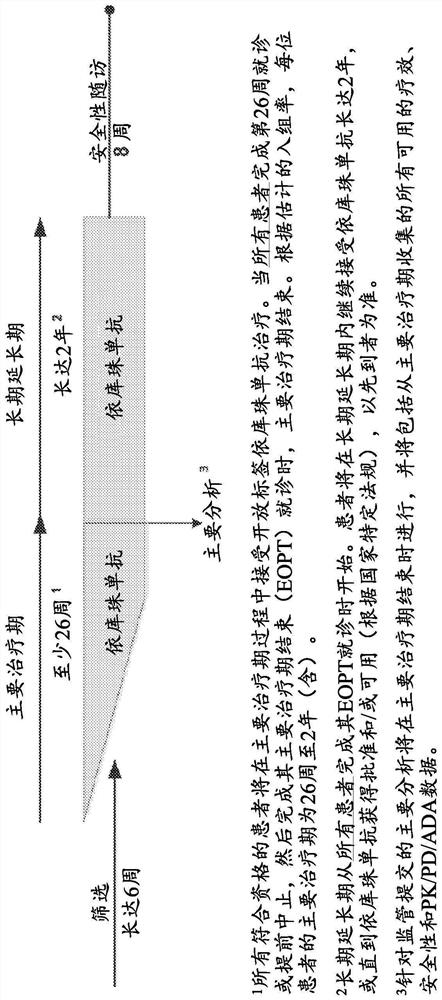
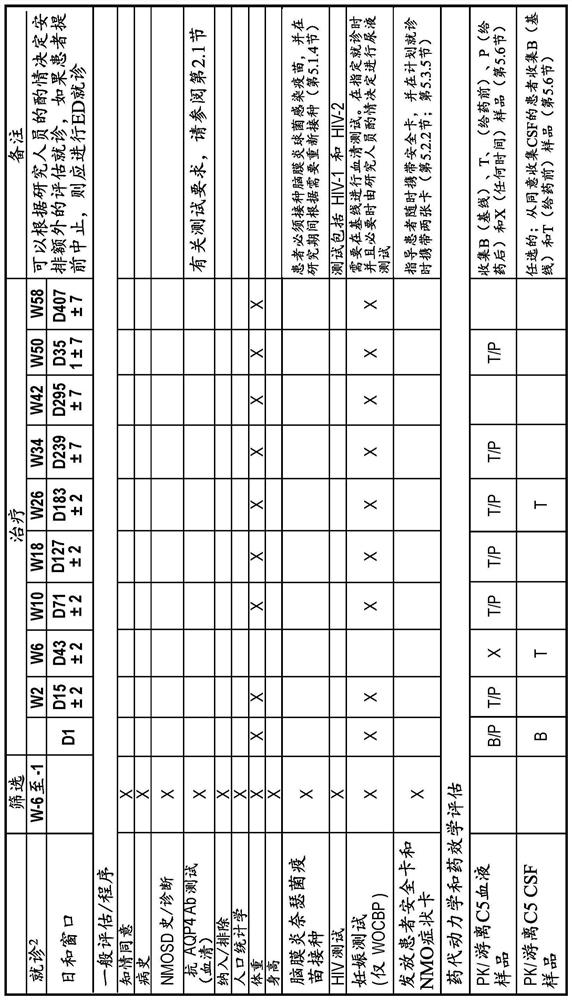
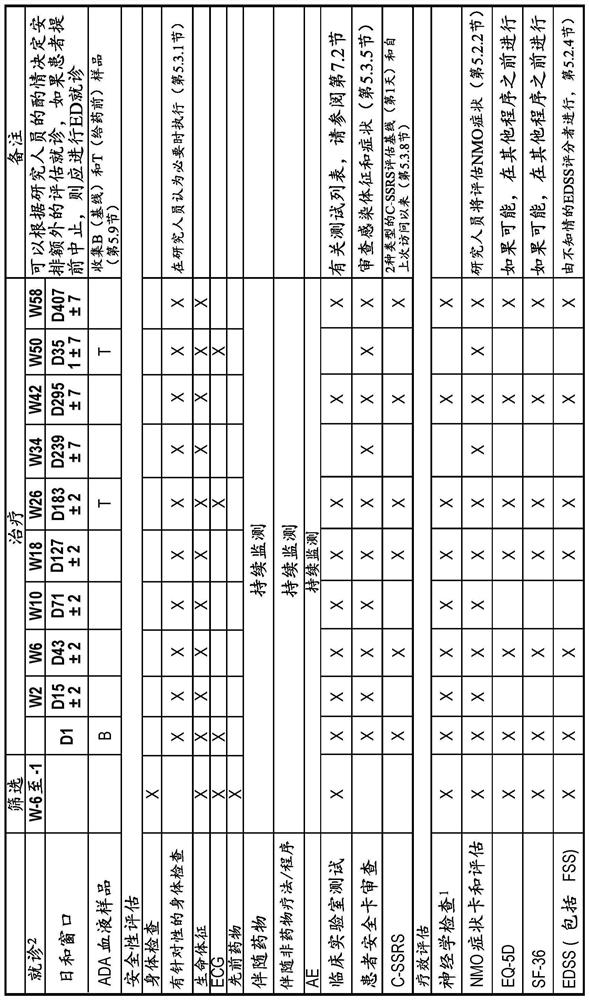
[0142] The study had 4 periods: the screening period, the main treatment period, the long-term extension period, and the safety follow-up period ( figure 1 ). During the screening period, patients are screened for eligibility for up to 6 weeks. When all patients completed the Week 26 visit or terminated early and then completed their end of primary treatment period (EOPT) visit, the primary treatment period ended and the long-term extension period began. All patients continued to receive eculizumab for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com