Camptothecin analogs and methods of preparation thereof
A compound and alkyl technology, applied in the field of new compounds and their preparation, can solve the problems of limitation, low solubility of camptothecin, poor anti-tumor activity, etc., and achieve the effect of improving activity and improving blood stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
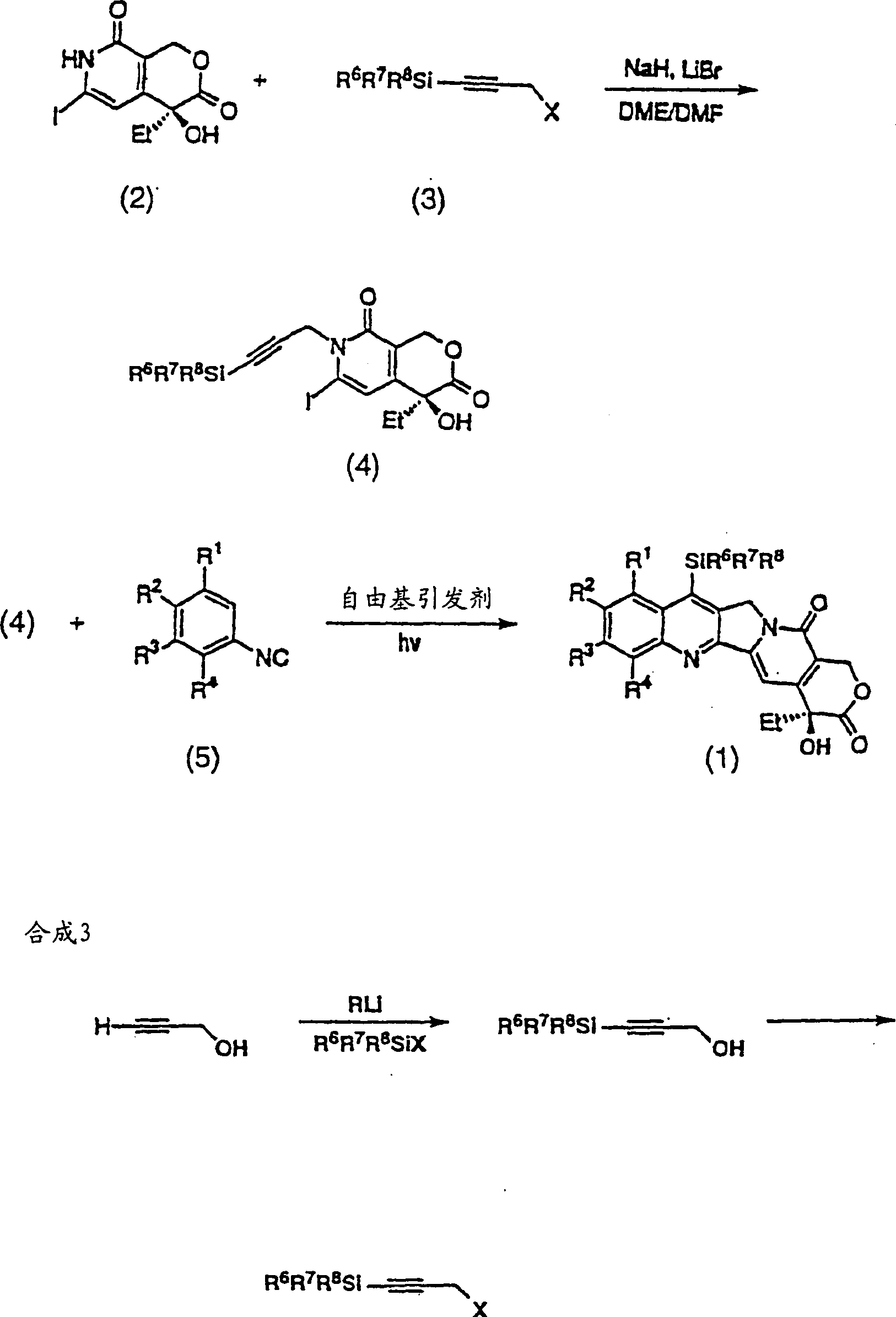
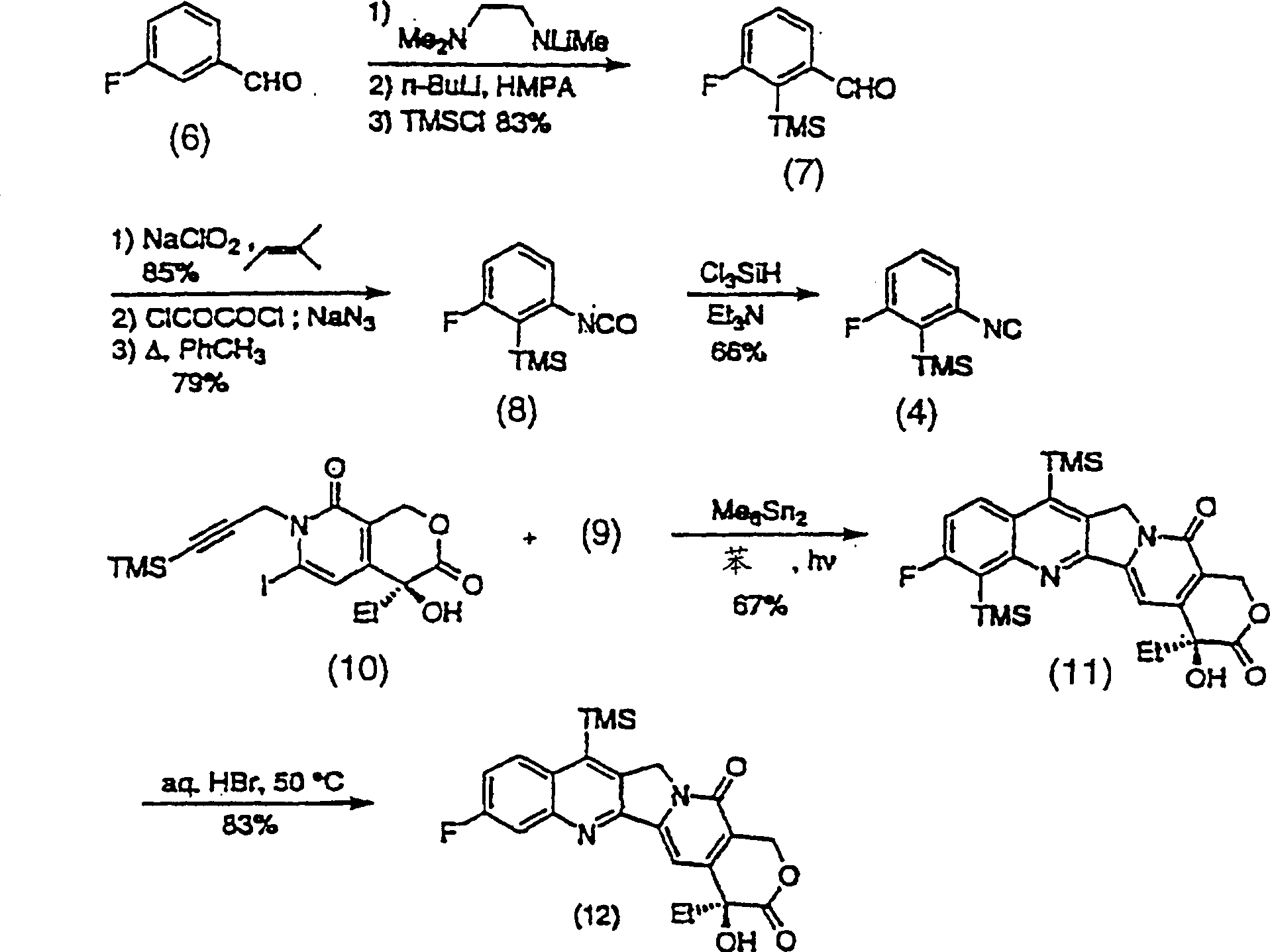
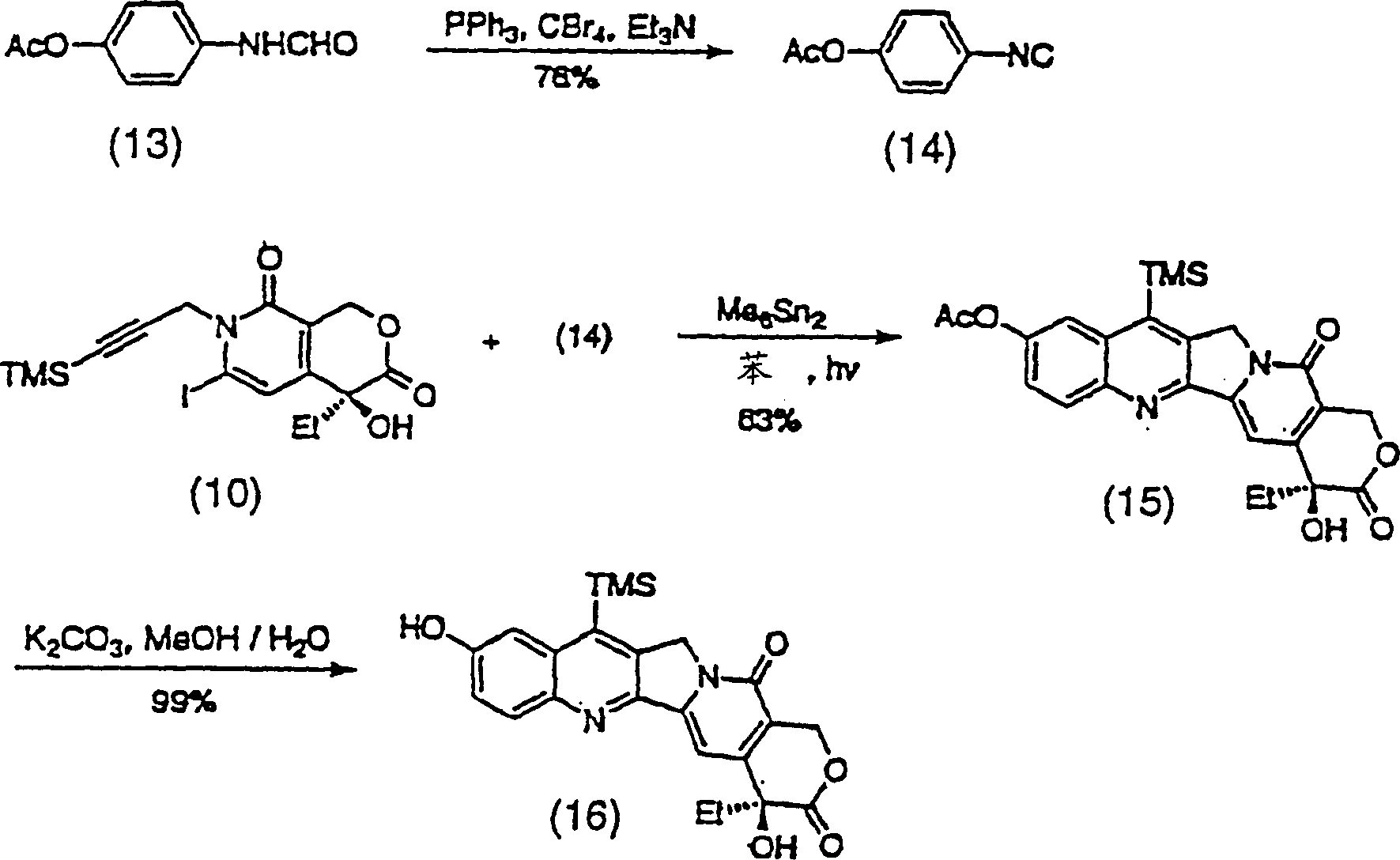
[0088] Figure 7-11 The preparation of compounds of formula (2) is shown. thus Figure 7 Three representative, novel A, B ring substituted silylcamptothecin compounds (36a), (36b) and (36c) are shown.
[0089] The first step in the synthesis of these analogs is the preparation of propargyl bromide (41), as Figure 8 shown. Trimethylsilylpropanal (38) can be obtained by Swern oxidation of the purchased trimethylsilylpropanol (37) with a yield of 85%. Sakar, T.K. et al., Tetrahedron 46, 1885 (1990). Aldehyde (38) was converted to dibromoalkene (39) in 55% yield following Procedure A of Corey, E.J. and Fuchs, P.L., Tetrahedron Lett., 36, 3769 (1972). Piers, E. and Gaval, A.V.J. Org. Chem., 55, 2374 (1990). 2 equivalents of n-BuLi were added in THF at -78°C, then warmed to 22°C and quenched with paraformaldehyde at reflux to give (40) in 84% yield. Finally, with anhydrous CH 2 Cl 2 Formulated triphenylphosphine and Br 2 The solution produced propargyl bromide (41) in 87%...
Embodiment 1
[0195] Preparation of (20S)-7-trimethylsilylcamptothecin
[0196]
[0197] (1) (S)-4-ethyl-4-hydroxyl-6-iodo-3-oxo-7-(3-trimethylsilyl-2-propynyl)-1H-pyrano[ 3,4-c]-8-pyridone
[0198] Under argon at 0°C, in (S)-4-ethyl-4-hydroxy-6-iodo-3-oxo-1H-pyrano[3 , 4-c]-8-pyridone [iodopyridone (2), 250 mg, 0.746 mmol] was added 60% NaH in mineral oil (31.3 mg, 0.783 mmol). After 10 minutes LiBr (150 mg, 1.75 mmol) was added. After 15 minutes at room temperature, 3-trimethylsilyl-2-propynyl bromide (430 mg, 2.24 mmol) was injected and the reaction mixture was heated at 65°C for 20 hours in the dark. The final solution was poured into brine (20ml), extracted with AcOEt (6 x 15ml) and dried (Na 2 SO 4 ). After removal of the solvent, the residue was subjected to flash chromatography (CHCl 3 / AcOEt 95:5) to give 283 mg (85%) of a foam:
[0199] [α] 20 D+36.7(c1, CHCl 3 ); IR(pure, cm -1 )3384, 2940, 2166, 1730, 1634, 1518, 1406, 1130, 841, 752; 1 H NMR (300MHz, CDCl 3 )δ0....
Embodiment 2
[0204] Preparation of (20S)-7-tert-butyldimethylsilylcamptothecin (DB-202)
[0205]
[0206] (1) (S)-4-ethyl-4-hydroxy-6-iodo-3-oxo-7-(3-tert-butyldimethylsilyl-2-propynyl)-1H- Pyrano[3,4-c]-8-pyridone
[0207] According to the process described in Example 1-(1), with iodopyridone (2) (200mg, 0.60mmol) and 3-tert-butyldimethylsilyl-2-propynyl bromide (280mg, 1.20 mmol) for reaction, flash chromatography (CH 2 Cl 2 / AcOEt 9:1), 173 mg (59%) of a white foam was obtained:
[0208] [α] D 20 +58(c0.2, CHCl 3 );IR(CHCl 3 , cm -1 )3548, 2950, 2927, 2859, 1745, 1648, 1526; 1 H NMR (300MHz, CDCl 3 )δ0.08(s, 6H), 0.92(m, 12H), 1.79(m, 2H), 3.77(brs, 1H), 5.00-5.25(m, 3H), 5.50(d, J=16.4Hz, 1H )7.19(s, 1H); 13 C NMR (75MHz, CDCl 3 )δ-4.9, 7.63, 16.6, 26.0, 31.6, 44.5, 66.3, 71.8, 89.4, 98.6, 100.0, 116.5, 118.1, 148.6, 158.0, 173.2; HRMS (EI) m / z C 19 h 26 INO 4 Si(M + ) calculated value 487.0679, measured value 487.0676; LRMS (EI) m / z 487 (M + ), 430, 386, 96, 81,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com