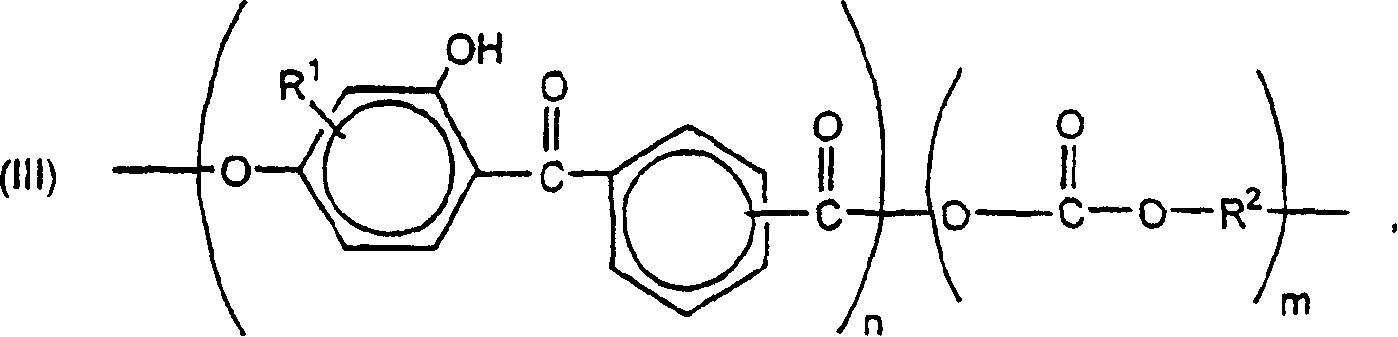
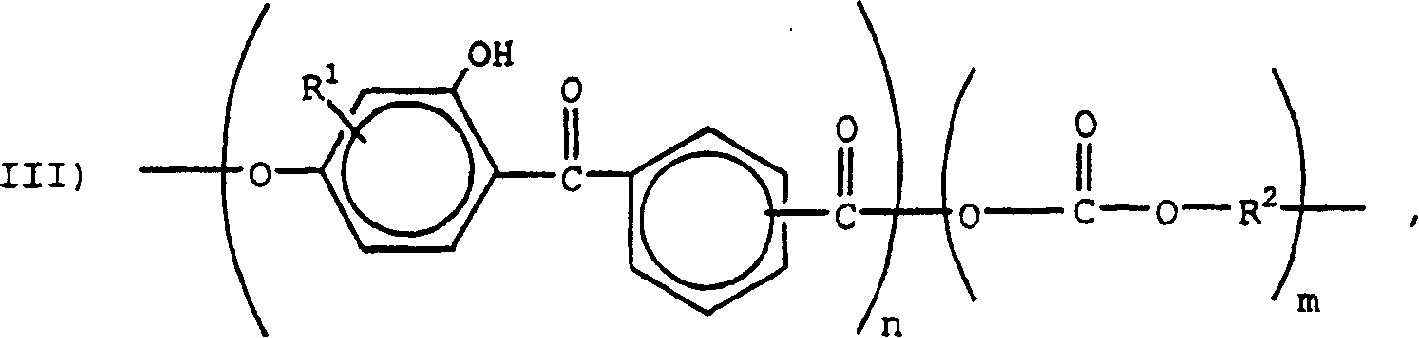
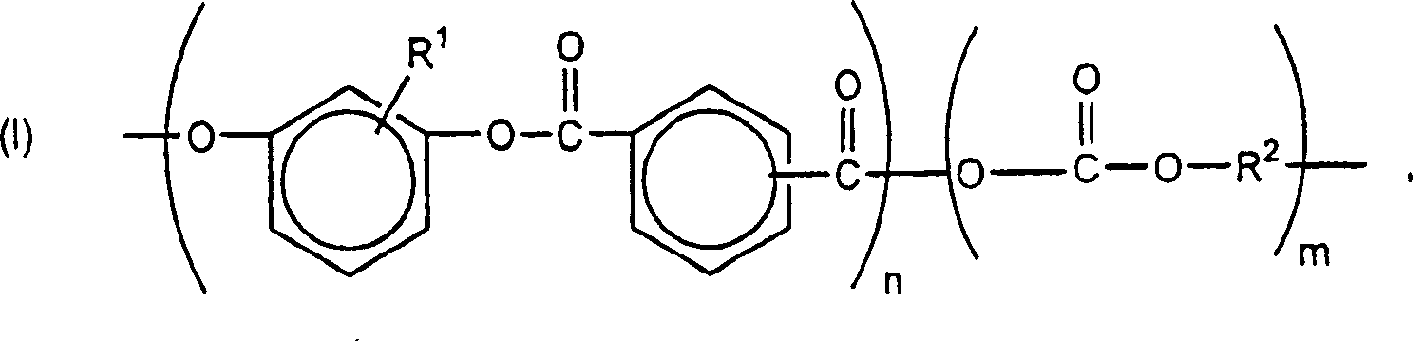
Weatherable block copolyestercarbonates and blends containing them
A technology of copolycarbonate and block copolymer, applied in the direction of machine/engine, transportation and packaging, mechanical equipment, etc., can solve the problem of not providing weather resistance in detail, and achieve the effect of improving weather resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
[0042] To several 1-liter four-necked flasks equipped with a mechanical stirrer, nitrogen inlet, reflux condenser, and two pressure-equalizing addition funnels, add 5 mmol of tetra-n-butylammonium bromide (TBAB) or methyltri-n-butyl chloride Ammonium (MTBAC), varying amounts of resorcinol and 150 ml of degassed dichloromethane. Then purging the flask with nitrogen, in each addition funnel, charge respectively 212mmol of 15% aqueous sodium hydroxide solution (maintained at 20°C and purging with nitrogen), and the m-phthalylene dissolved in 100ml of dichloromethane Various degassed mixtures of acid chloride and terephthaloyl chloride.
[0043] The sodium hydroxide solution was added to the flask under a nitrogen atmosphere while stirring, and the resorcinol was subsequently dissolved to form a translucent two-phase mixture. The isophthaloyl / terephthaloyl chloride mixture was then added and stirring continued followed by an exotherm leading to weak reflux. Stirring was continue...
Embodiment 11
[0049] A sample of the product of Example 6 (2 g) and several other materials described below were dissolved in methylene chloride or chloroform (8 ml) and a film about 250 μm thick was applied to a glass plate with a doctor blade. The solvent was allowed to evaporate leaving a film approximately 40 [mu]m thick, which was floated from the glass plate with water. The haze value of the obtained film was measured with a Gardner XL-835 haze meter.
[0050] The results obtained are shown in Table II. The following other materials were evaluated; commercially available bisphenol A polycarbonate (PC), a resorcinol polycarbonate with a 1:1 ratio of isophthalate to terephthalate groups and a molecular weight of approximately 50,000 Aryl compound (RPA), a blend of PC with the product of Example 6, and two PC-RPA blends.
[0051] film material
[0052] These results illustrate the improved miscibility of the blend of block copolycarbonate and polycarbonate of Example 6 compar...
Embodiment 12~16
[0054] Various amounts of resorcinol and triethylamine and 100ml of dichloromethane were added to a 1-liter three-necked flask equipped with a reflux condenser, a mechanical stirrer and a pressure-equalizing addition funnel. The flask was protected with nitrogen, and a solution of 10.151 g (50 mmol) of isophthaloyl chloride and terephthaloyl chloride in 150 ml of dichloromethane was added dropwise over 4 to 8 minutes, so as to maintain a steady reflux. The mixture was stirred at reflux temperature for an additional 30 min, then transferred to a separatory funnel, washed once with water, twice with dilute hydrochloric acid and once with water.
[0055] The oligomer solution was transferred to a phosgenation reactor similar to that of Examples 1-10. After addition of bisphenol A, triethylamine (1 mol % based on bisphenol A), water and p-cumylphenol as chain terminator, the phosgenation reaction was carried out as described in the example .
[0056] The results obtained and pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com