A method of electrophoresis
An electrophoretic separation and charge technology, applied in the field of separation of protein and/or peptide components by electrophoresis, can solve problems such as unsatisfactory solutions to this problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
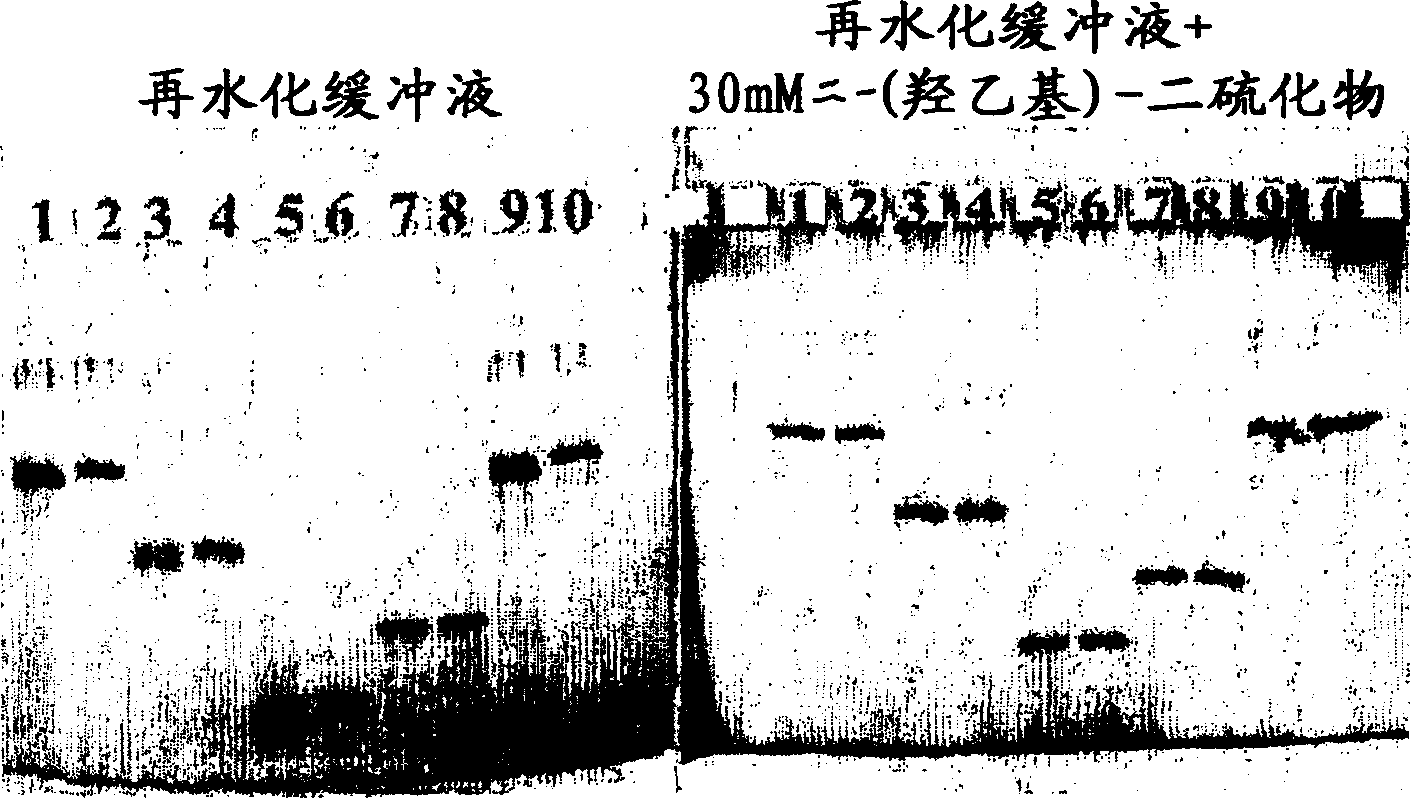
[0129] Clean Gel TM 25 (Amersham Biosciences, Uppsala, Sweden), a commercially available dry gel for zone electrophoresis, with a size of 250×110×0.5 mm (after rehydration), prepared by T=5% (5 g Monomer / 100ml) and C=3% (3 g crosslinker / 100 g monomer) stacking gel and T=10 and C=2 separating gel, which were cut in half. One half was rehydrated in a rehydration solution delivered by gel containing 0.3M Tri-acetic acid, pH 6.5 buffer and 0.1% SDS. The other half was rehydrated in the corresponding solution to which bis(hydroxyethyl)disulfide was also added at a concentration of 300 mM. Place the two gel halves in a MultipHor at 15°C TM on a cooling plate (Amersham Biosciences, Uppsala, Sweden). The cathode paper core contains Tris-ribinoic acid-SDS, and the anode paper core contains Tris-acetic acid (electrode solution delivered by gel). Dissolve 0.3 mg / ml of bovine serum albumin, chicken ovalbumin, soybean trypsin inhibitor and bovine carbonic anhydrase in 0.375M Tris / HCl,...
Embodiment 2
[0133] Immobiline DryStrip, pH 6-11, 18 cm long TM , rehydrated overnight with a solution containing 8M urea, 0.5% CHAPS, 1% IPGTM buffer, pH 6-11, and the redox chemicals defined below. Samples extracted from mouse liver containing 50 mM Tris, 7 M urea, 2 M thiourea, 4% (w / v) CHAPS, and 10 mM DTT were diluted with the same solution used for rehydration (10 μl diluted to 160 μl) to provide the final concentration A sample of 1 mg / ml. 80 μl of each sample, corresponding to the rehydrated IPG-strip, was applied to the sample cup adjacent to the anode end of the strip. Silver staining of gels generated in the second dimension is shown in figure 2 a-f.
[0134] Fig. Sample and rehydration solution
[0135] a 8M urea, 0.5% CHAPS, 1% IPG buffer pH6-11, 20mM mercaptoethanol
[0136] b 8M urea, 0.5% CHAPS, 1% IPG buffer pH6-11, 20mM dithiothreitol
[0137] c 8M urea, 0.5% CHAPS, 1% IPG buffer pH6-11, 50mM bis-(2-hydroxyethyl)-disulfide
[0138] d 8M urea, 0.5% CHAPS, 1% IPG buf...
Embodiment 3
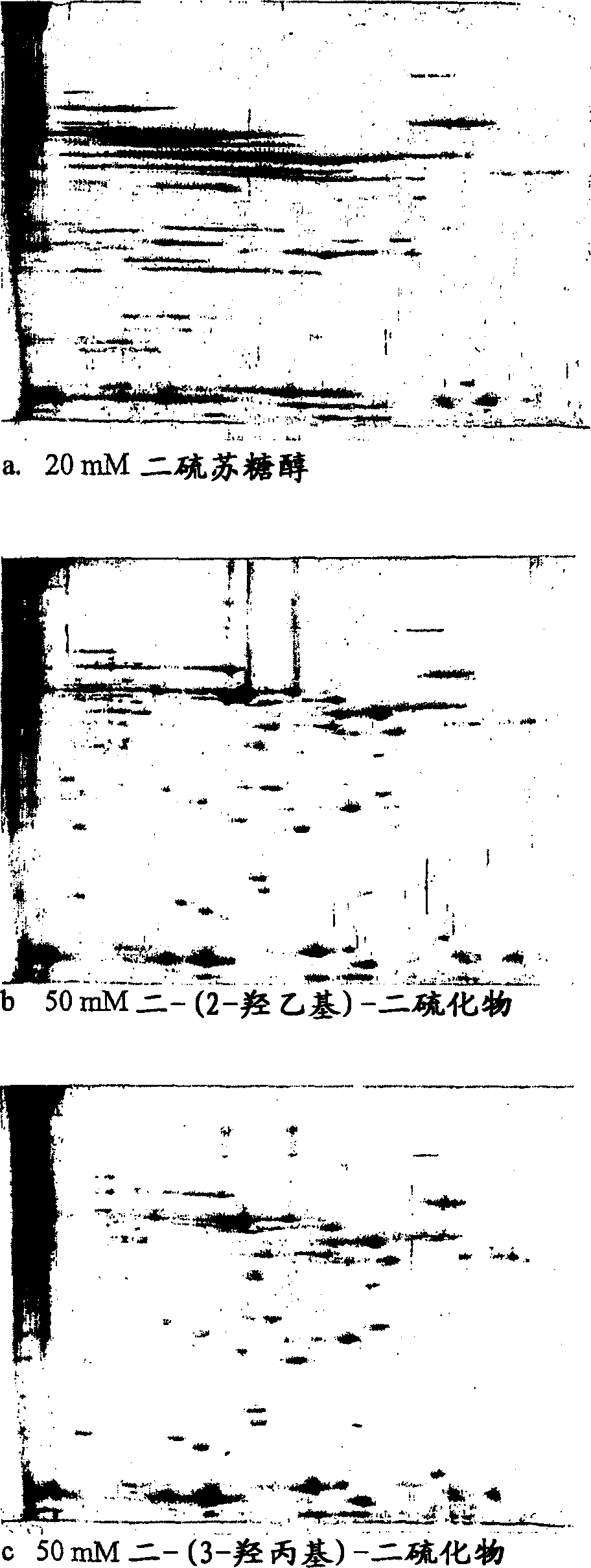
[0143] Immobiline DryStrip pH 7.5-9.5, 24 cm long TM , rehydrated overnight with a solution containing 8M urea, 0.5% CHAPS, 1% IPGTM buffered pH 6-11 and redox chemicals as described below. Samples containing murine liver proteins were diluted with the same solution used for rehydration (10 μl diluted to 160 μl) to obtain samples with a final concentration of 1 mg / ml. 80 μl of each sample, corresponding to the rehydrated IPG-strip, was applied to the sample cup adjacent to the anode end of the strip. The gels produced in the second dimension were silver-stained and the results are shown in panels h–j.
[0144] Fig. Sample and rehydration solution
[0145] H 8M urea, 0.5% CHAPS, 1% IPG buffer pH8-10.5, 20mM mercaptoethanol
[0146] I 8M urea, 0.5% CHAPS, 1% IPG buffer pH8-10.5, 50mM bis-(2-hydroxyethyl)-disulfide
[0147] J 8M urea, 0.5% CHAPS, 1% IPG buffer pH8-10.5, 50mM bis-(3-hydroxypropyl)-disulfide
[0148] This experiment shows that the addition of disulfides accord...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com