Bacterial vaccine strain quality control method
A technology for strains and vaccines, applied in measuring devices, material analysis by electromagnetic means, instruments, etc., can solve problems such as quality control of unseen bacterial vaccine strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 1
[0022] Embodiment 1 general method
[0023] 1. Preparation of Bacterial PFGE Blocks
[0024] Take 10ml of the Leptospira vaccine strain grown in rabbit serum phosphate medium at 28°C for 7-10 days in a centrifuge tube, balance, centrifuge at 10,000rpm at 4°C for 20min, discard the supernatant, add 1ml of buffer solution (containing 1MTris, 0.5 MEDTA and 5M NaCl solution), transferred to a 1.5mleppendorf tube, and centrifuged at 6000rpm for 5 minutes. Pour off the supernatant. Add 400 μl of buffer solution according to the concentration of Leptospira bacteria to dissolve the precipitate and mix well, take 200 μl of bacterial suspension and place it in a 1.5 mleppendorf tube, and incubate at 37°C for 5 minutes. Add an equal amount of 1% gold medal agarose solution (purchased from Lonza Company, USA), mix well, add to the mold, and solidify at room temperature for about 15 minutes to obtain bacterial PFGE gel blocks.
[0025] 2. Treatment of Bacterial PFGE Blocks
[0026] Put...
Embodiment 2
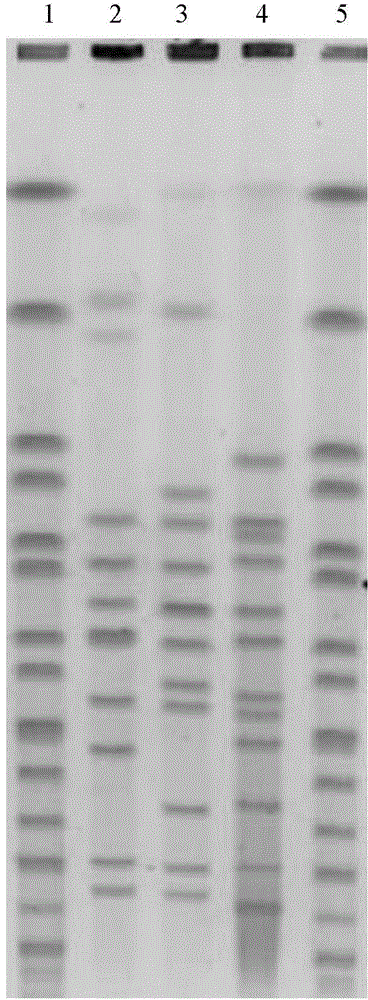
[0030] Carry out the PFGE analysis method as described in Example 1 to the following Leptospira vaccine strains (all from Wuhan Institute of Biological Products Co., Ltd.): MarkerH9812; Jaundice and hemorrhagic group (Lai strain); Autumn group (Lin 4 strains) ; Influenza typhoid group (6 strains); MarkerH9812, obtained figure 1 The results shown.
[0031] This result shows that the number, size and distribution of DNA fragments of Leptospira vaccine strains of different serogroups are different, showing different PFGE patterns.
Embodiment 3
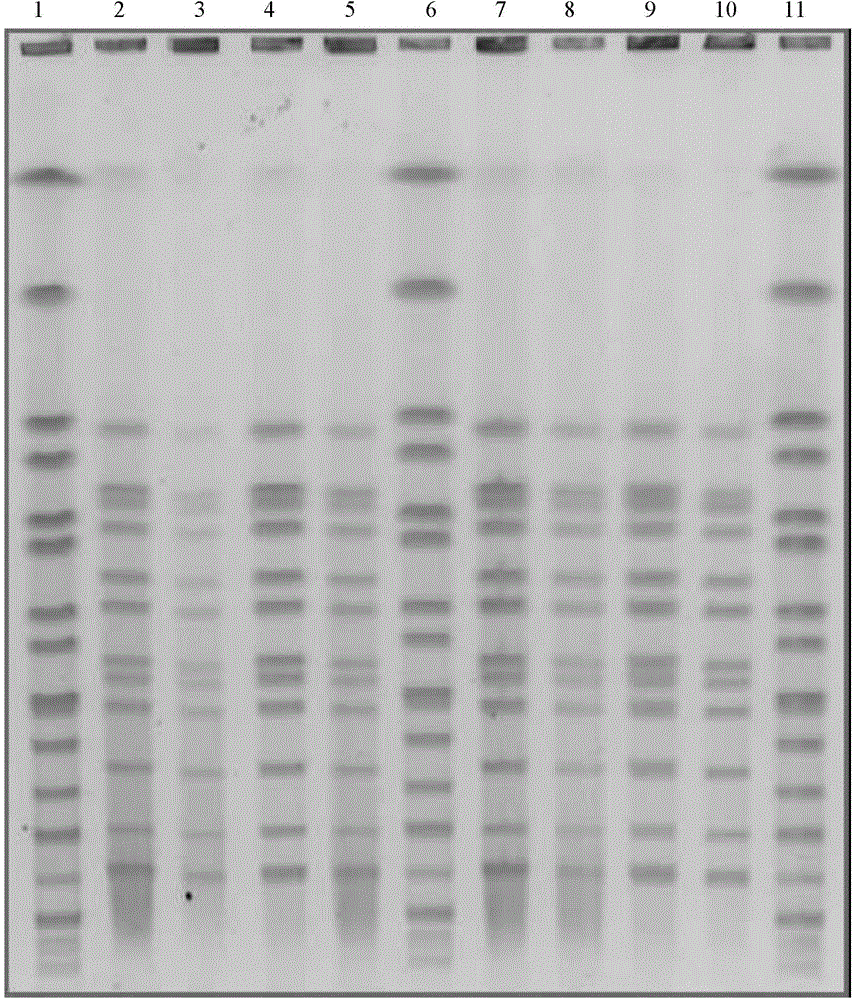
[0033] Different passages of a Leptospira vaccine strain influenza typhoid group (Lin 6 strains) (Leptospira Lin 6 strains were obtained from Wuhan Institute of Biological Products Co., Ltd. before animal passage and through animal passage; then The Leptospira strain is carried out one generation every 7 days or so on the in vitro artificial culture medium-rabbit serum phosphate medium, totally 20 generations) Carry out the PFGE analysis method as described in Example 1, obtain figure 2 The results shown.
[0034] This result shows that the DNA bands of the influenza typhoid group (Lin 6 strain) are completely consistent before animal subculture and within 20 generations after animal subculture, and have the same PFGE pattern. The culture characteristics and growth morphology of these different generations of strains on the rabbit serum phosphate medium were the same, and the results of serotype analysis of the strains of different generations were also consistent, which furt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com