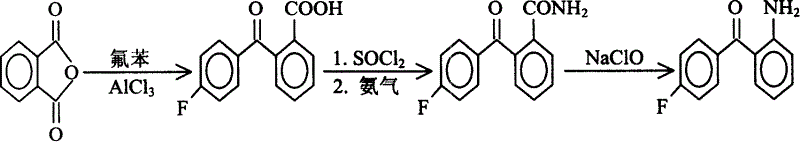
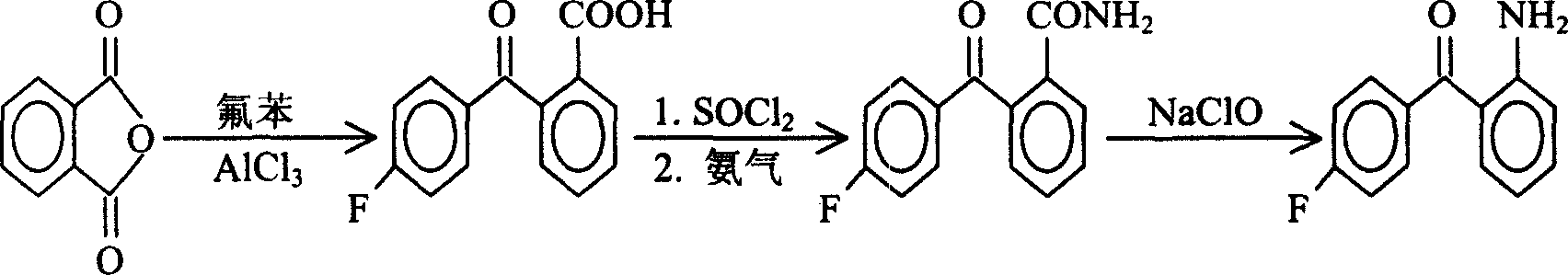
Process for preparing 2-amino-4'-fluoro-benzophenone
A technology of benzophenone and fluorobenzoyl benzoic acid is applied in the field of preparation of 2-amino-4'-fluoro-benzophenone, and can solve the problem of harsh reaction conditions, long reaction steps, and side-by-side reactions. Over-reacting issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The following examples illustrate the specific implementation method of the present invention
[0021] The first step: the preparation of 2-p-fluorobenzoylbenzoic acid
[0022] Add 25g (0.17mol) of phthalic anhydride and 50g (0.375mol) of anhydrous aluminum trichloride to 120g (1.25mol) of fluorobenzene, the reaction occurs immediately, and hydrogen chloride gas is released, which is absorbed by water. At this time, the reaction liquid is Orange yellow, slowly heated to 55°C-70°C, the reaction proceeds violently, the reaction solution gradually turns dark red, keep warm for 4 hours, cool to room temperature, slowly pour the reaction solution into ice water filled with hydrochloric acid while stirring, and put The reaction solution was adjusted to PH1, the organic layer was separated, washed with water, the excess fluorobenzene was recovered under reduced pressure, cooled to room temperature, a large amount of solids were precipitated, 40 g of white powder was obtained a...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap