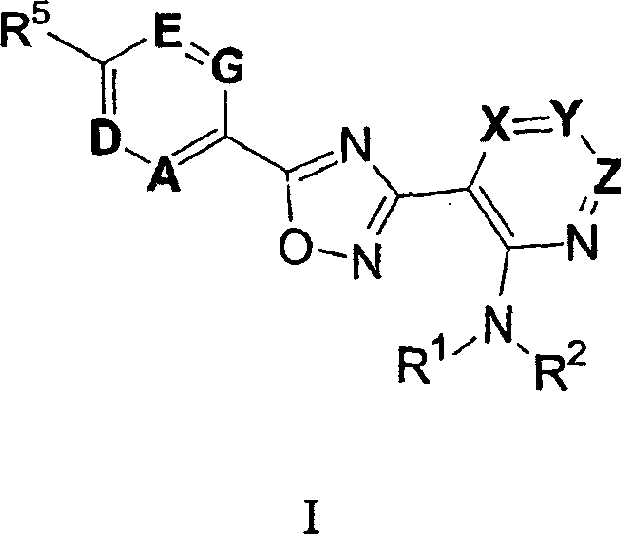
3-(2-amino-1-azacyclyl)-5-aryl-1,2,4-oxadiazoles as S1P receptor agonists
An alkyl, methyl technology, applied in the field of 3-(2-amino-1-azacyclyl)-5-aryl-1,2,4-oxadiazole as an S1P receptor agonist, Ability to resolve issues such as restricted use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0412] 3-(2-(N-methylamino)pyridin-3-yl)-5-((2-methylpropyl)phenyl)-1,2,4-oxadiazole
[0413] Step A: 3-(2-(Chloro)pyridin-3-yl)-5-(4-(2-methylpropyl)phenyl)-1,2,4-oxadiazole
[0414] 500mg (2.8mmol) 4-(2-methylpropyl) benzoic acid, 600mg (3.1mmol) 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride and a mixture of 420 mg (3.1 mmol) 1-hydroxybenzotriazole (0.42 g, 3.09 mmol) in 10 mL DMF was stirred at room temperature for 10 minutes. N-Hydroxyamidine 1 (620 mg, 3.6 mmol) was added and the resulting mixture was heated at 20°C for 3 hours. The reaction was cooled and concentrated. Purification by silica gel chromatography using 3:1 v / v hexane / EtOAc as eluent afforded 103 mg of the title compound:
[0415] 1 H NMR (500MHz, CDCl 3 )δ8.56 (dd, J=2.0, 4.8, 1H), 8.38 (dd, J=2.1, 7.6, 1H), 8.12 (d, J=8.2, 2H), 7.42 (dd, J=4.8, 7.6, 1H), 7.35(d, J=8.2, 2H), 2.59(d, J=7.1, 2H), 1.94(m, 1H), 0.94(d, J=6.7, 6H); ESI-MS 314.1(M+ H).
[0416] Step B: 3-(2-(N-Methylamino)...
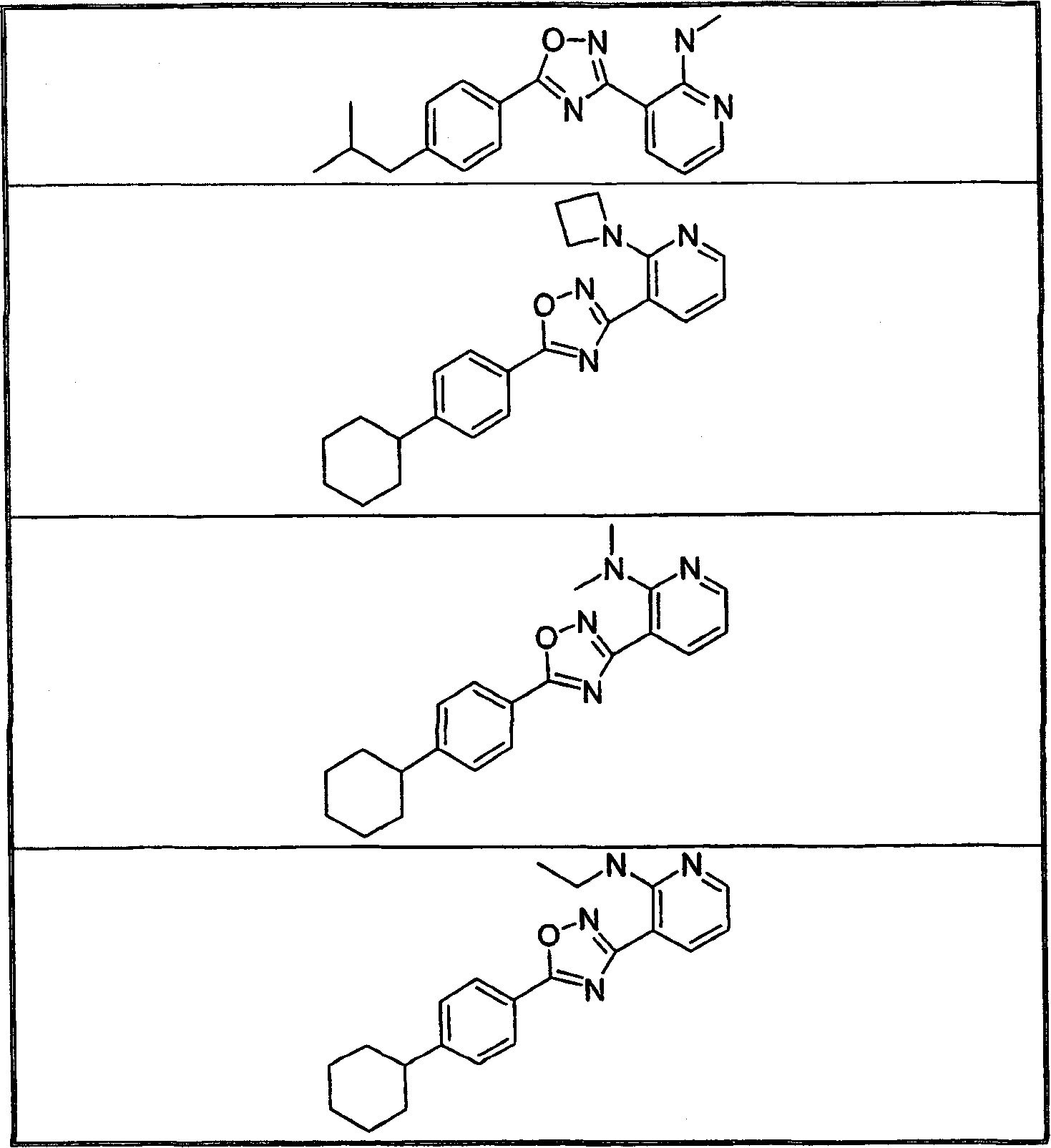
Embodiment 2-9
[0420] The following compounds were followed in a manner similar to that described in Example 1, but using 4-(cyclohexyl)benzoic acid in Step A instead of 4-(2-methylpropyl)benzoic acid and the appropriate N-hydroxyamidine N-Hydroxyamidine 1, prepared in Step B by substituting the appropriate amine for N-methylformamide.
[0421]
[0422]
[0423]
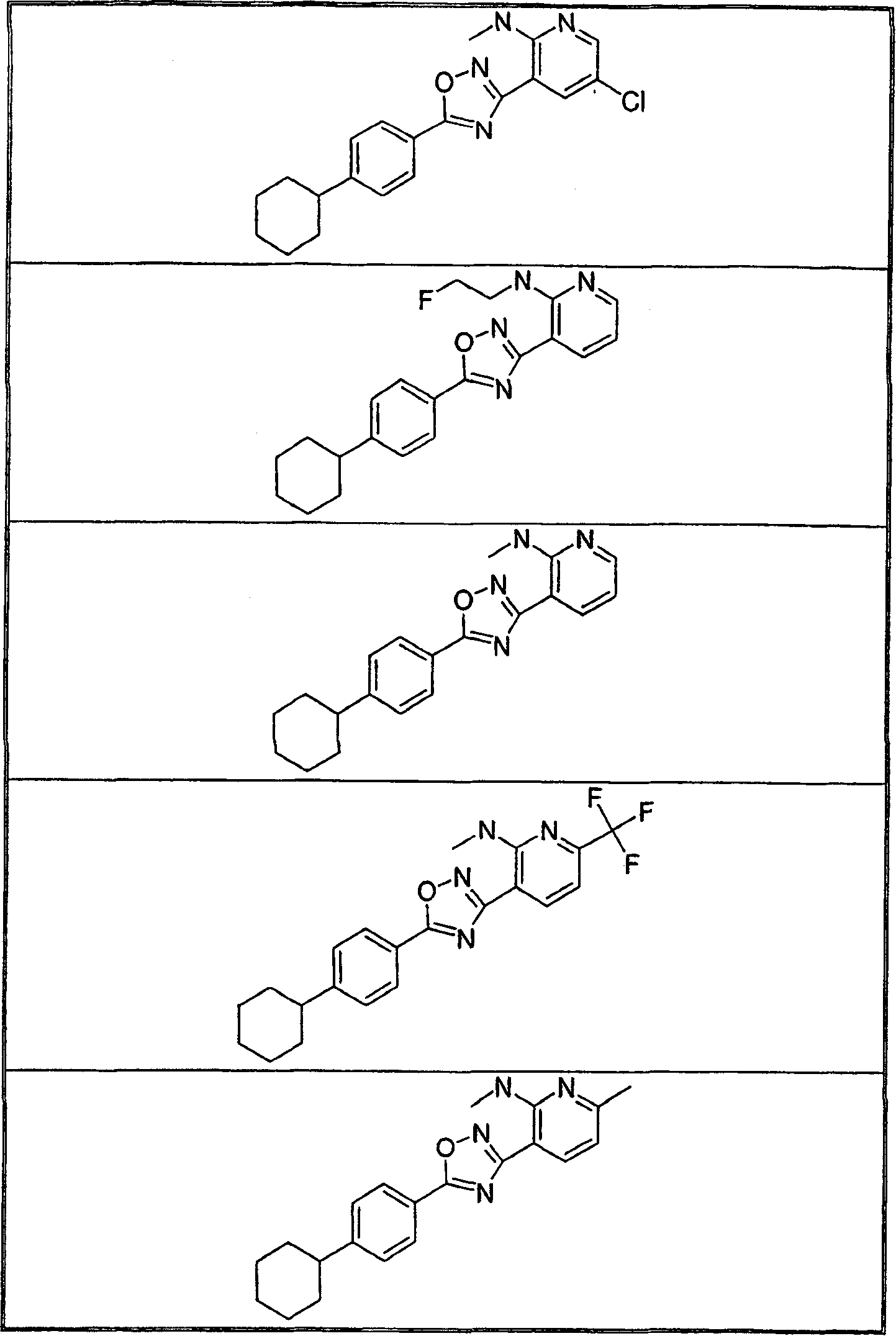
Embodiment 10-13
[0425] The following compounds were followed in a manner similar to that described in Example 1, but in Step A the appropriate carboxylic acid was used instead of 4-(2-methylpropyl)benzoic acid and N-hydroxyamidine 3 was used instead of N-hydroxyamidine 1 And made.
[0426]
[0427]
[0428]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com