Treatment of infectious diseases
A disease, anti-infective agent technology, applied in the field of chemosensitizing compounds, can solve problems without mentioning anti-infection or anti-drug resistance activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
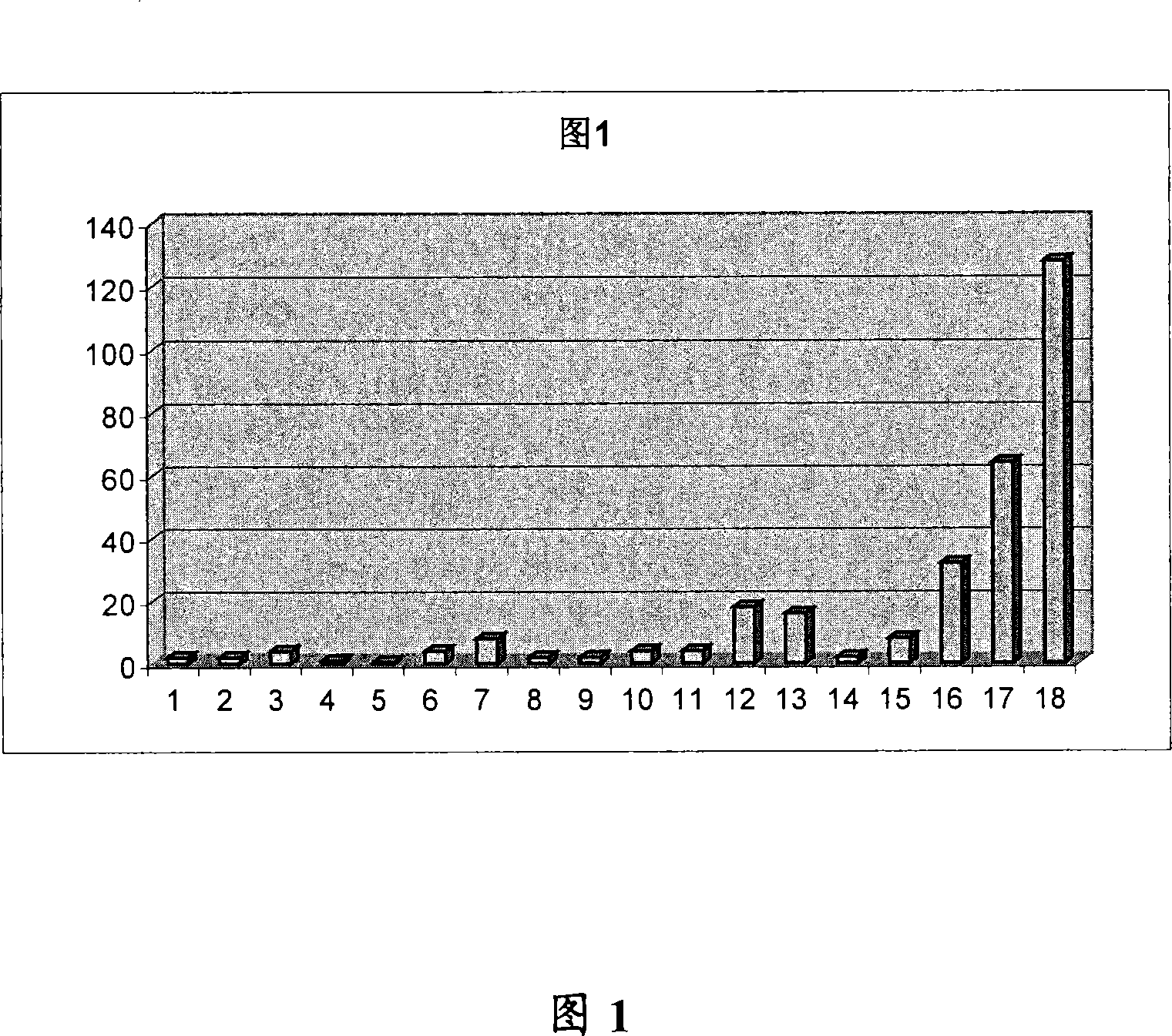
[0227] The influence of embodiment 1-modification promethazine
[0228] Table 1 shows that with different R 1 , R 2 , R 7 and R 8 Structures, MIC values and DR ratios of a series of promethazine derivatives with substituents.
[0229] The anti-infective agent used was ciprofloxacin, and the bacterial strain was Escherichia coli LN 3164.
[0230] Chemosensitizing compounds (promethazine and its derivatives) have the following general structures:
[0231]
[0232] R 1
R 2
R 7
R 8
name
Mic
(μg / ml)
DR ratio
h
Cl
h
h
h
h
h
h
h
Cl
Cl
Cl
S-CH 3
CF 3
h
h
h
Oh
Oh
h
h
h
h
h
h
Oh
h
h
promethazine
1-Chlorpromazine
Chlorpromazine
7-Hydroxychlorpromazine
7,8-Dihydroxychlorpromazine
Methiopromazine
Triflupromazine
64
64
32
1...
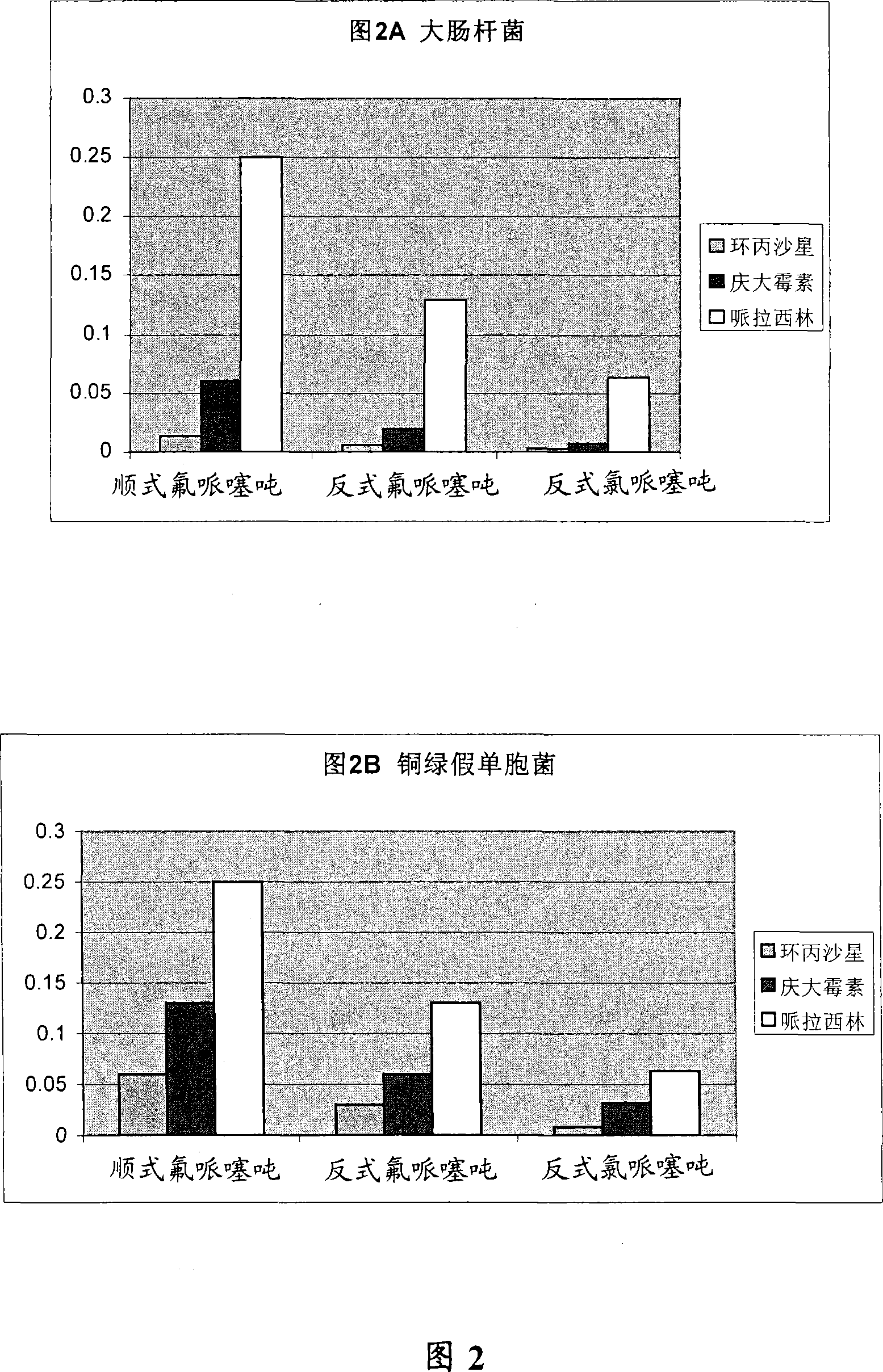
Embodiment 2
[0246] Example 2 - Effect of Stereochemistry
[0247] name
Mic
(μg / ml)
DR ratio
trans-flupenthixol
cis-flupenthixol
trans-clopenthixol
32
32
16
64
32
128
[0248] Table 4
[0249] The above results indicate that stereoisomeric configuration is necessary for optimal anti-DR activity. For example, trans-flupenthixol is a more potent anti-DR agent than cis-flupenthixol, which is the most potent anti-DR agent. Thus, the orientation of the side chain amines relative to the tricyclic core appears to be an important determinant of anti-DR activity.
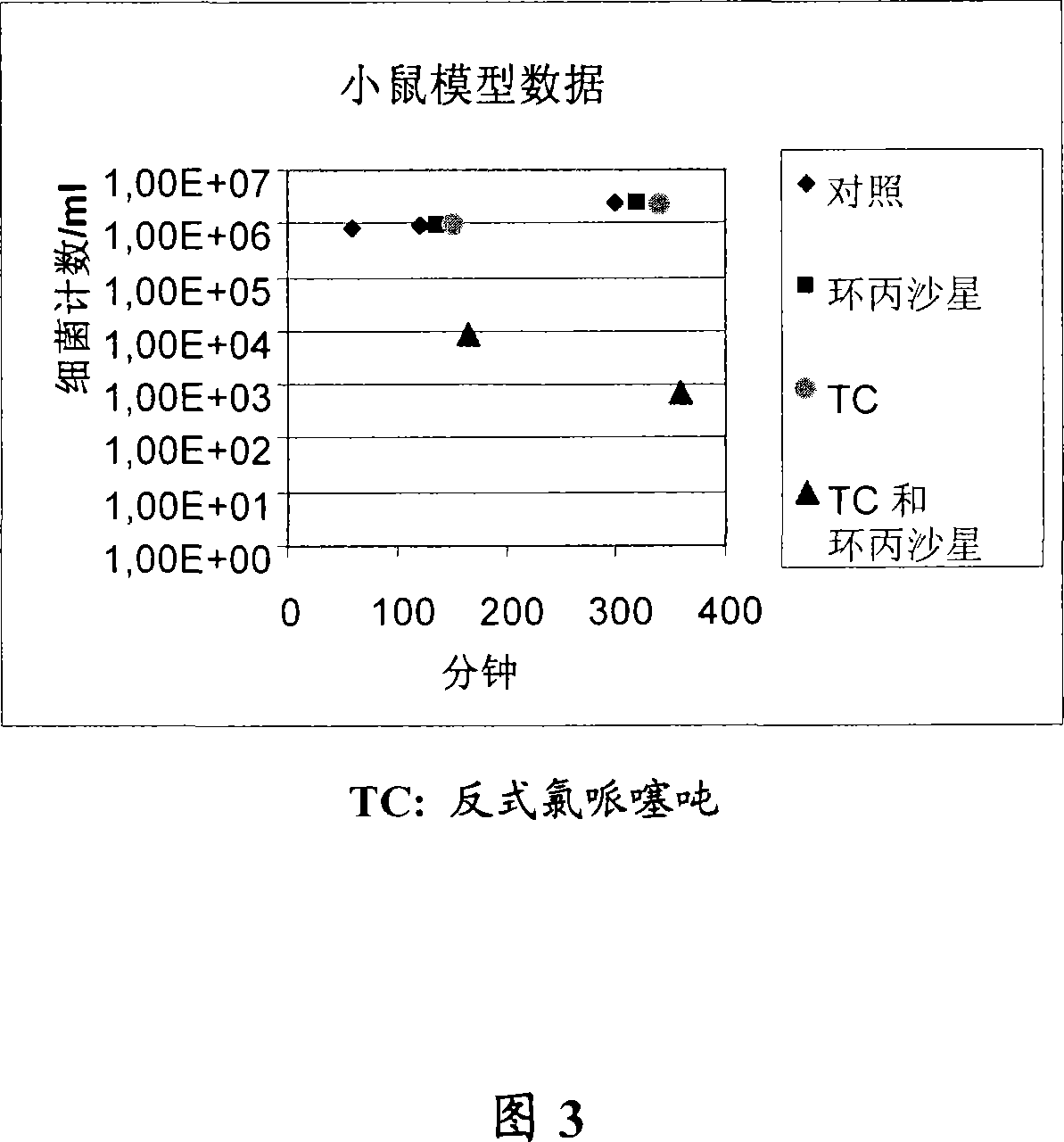
Embodiment 3
[0250] Example 3 - Effect of Hydrophobicity
[0251] To determine whether the differences in anti-DR potency among side chain-altered chemosensitizer compounds were also due to changes in overall hydrophobicity, the octanol:buffer partition coefficients for each drug in Tables 1, 2, 3, and 4 were compared with their The DR ratios were compared. No statistically significant correlation (P > 0.5) was found between hydrophobicity and anti-DR activity (data not shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com