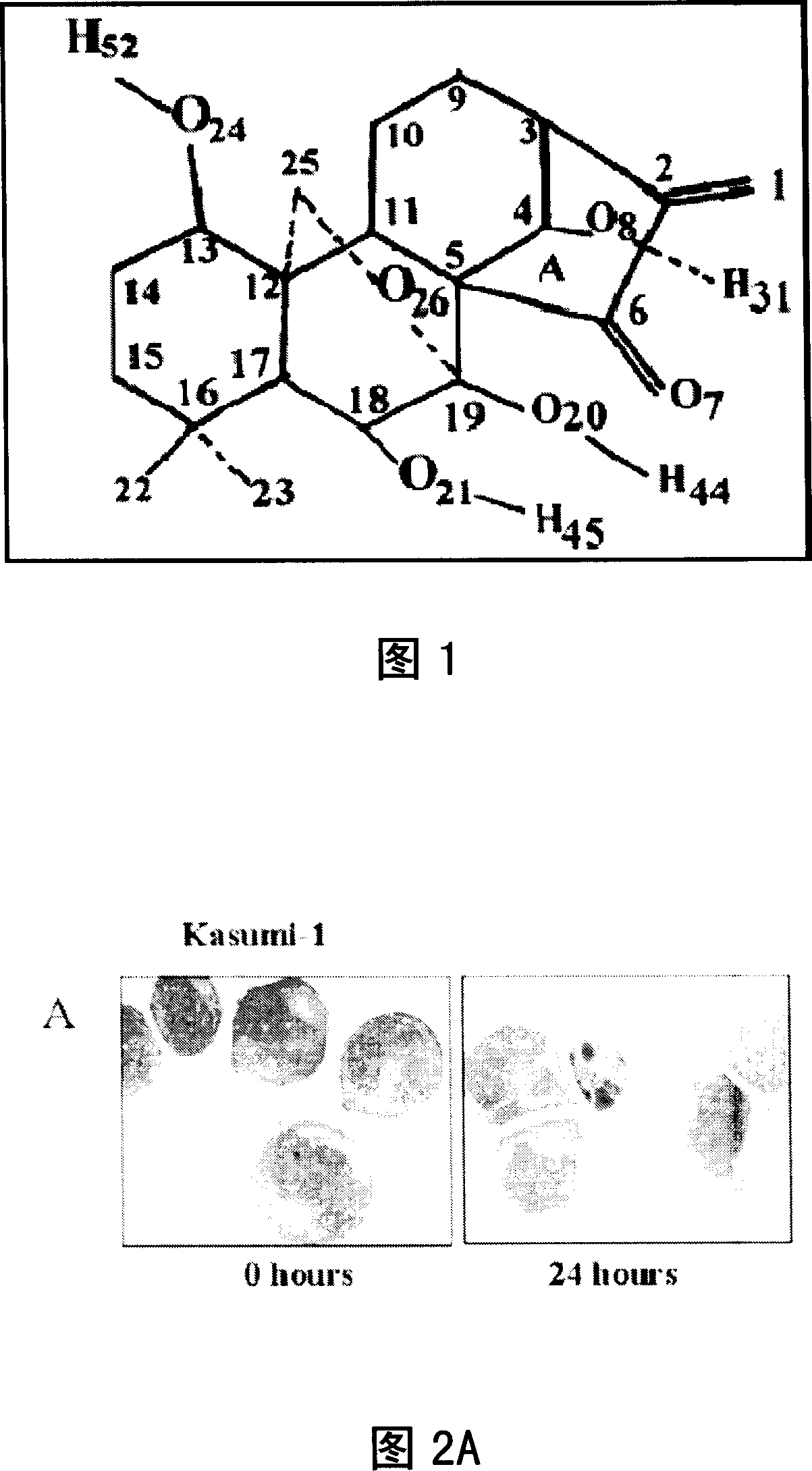
Application of oridonin in medicine preparation
A technology of Rubescensin A and medicine, which is applied in its application field in the field of pharmacy, can solve the problems that there are no animal models or clinical experiment data of Rubescensin A recorded, and achieve good medicinal prospects and strong pharmacological effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Oridonin A Induced Apoptosis of t(8;21) Leukemia Cells in Vitro
[0058] 1. Materials and methods
[0059] 1. Reagents and Instruments
[0060] The purity of oridonin is 98%-99.8%. The stock solution prepared by dissolving in DMSO (Sigma) to a concentration of 10-2 mol / L was stored at -20°C. Propidium iodide (PI) was a product of Sigma, USA. Propidium iodide was made into a 250 μg / mL stock solution with triple distilled water and stored at 4°C in the dark. Fetal bovine serum was purchased from Hyclone Company, USA. Annexin V detection kit (ApoAlert Annexin-V kit) was purchased from BD Biosciences, USA. The flow cytometer is a product of Beckman Coulter, USA. Fluorescence microscope (Olympus, BX60) and phase contrast microscope (Olympus, IMT) were purchased from Japan Olympus Company. Cytospin, a cell smear instrument, was purchased from Shandon Company, UK.
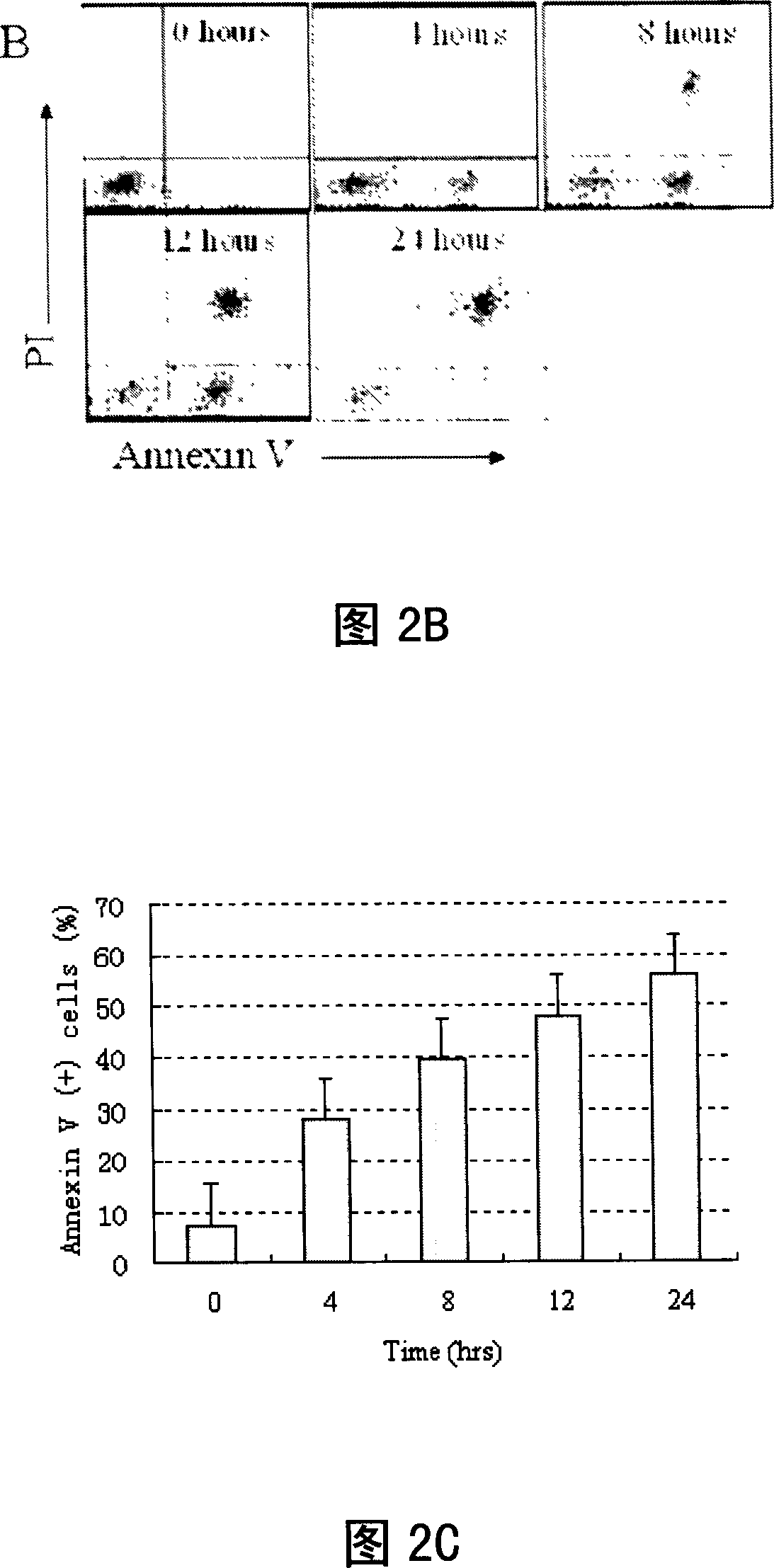
[0061] 2. Cell culture and treatment, cell morphology observation
[0062] Human t(8;21) leukemia cell l...
Embodiment 2
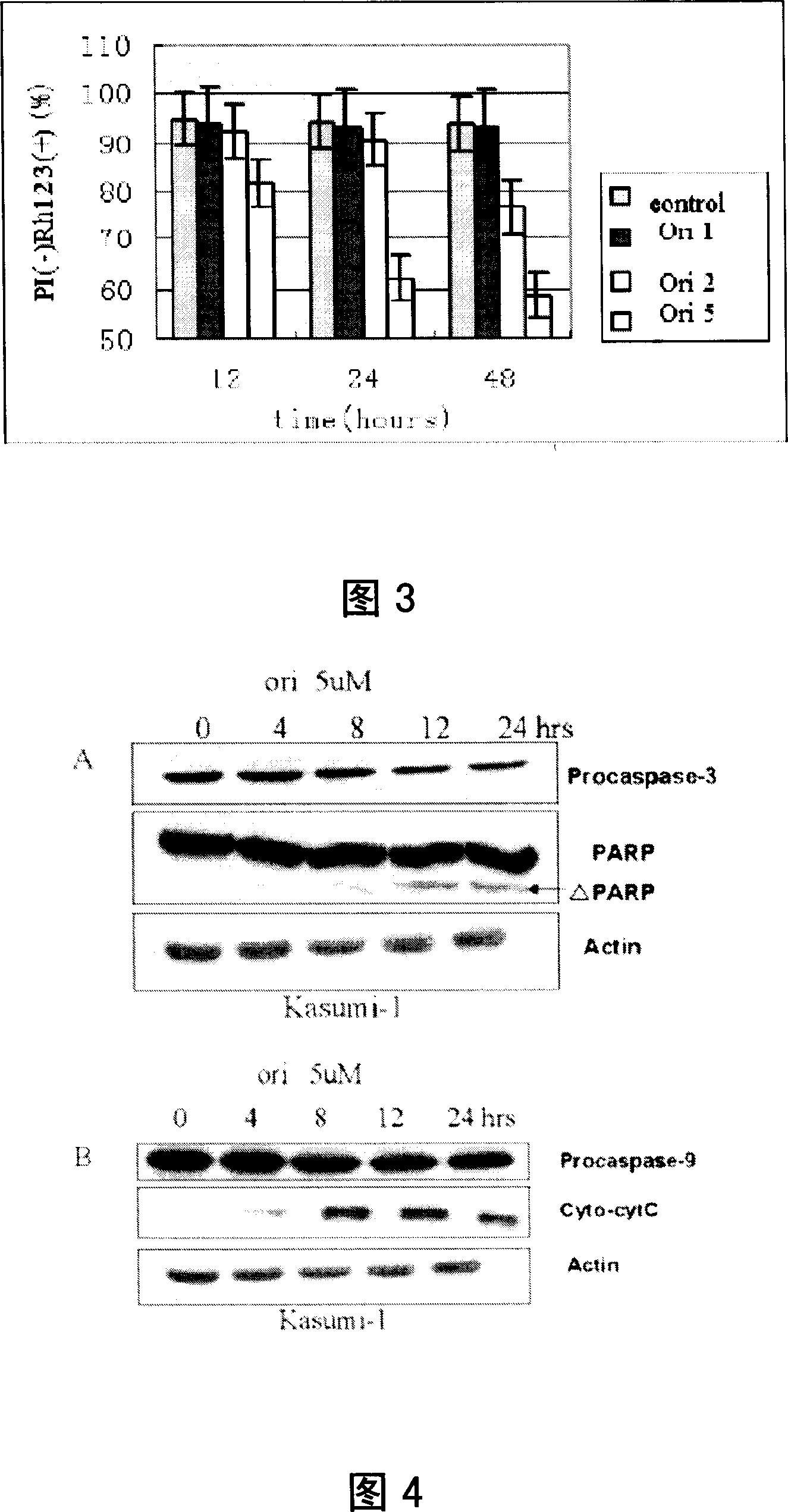
[0072] Molecular Mechanism of Oridonin A Inducing Kasumi-1 Cell Apoptosis
[0073] 1. Materials and methods
[0074] 1. Reagents and Instruments
[0075] For Rubescensine A, propidium iodide, and fetal bovine serum, see Example 1 "Materials and Methods". Rhodamine (Rhodamine, Rh123) is a product of Sigma Company in the United States. Rhodamine was made into a 10 μg / mL stock solution with triple-distilled water and stored at 4°C in the dark. Caspase-3 specific inhibitor Z-DQMD-FMK is a product of Sigma Company in the United States, dissolved in DMSO and stored at -20°C before use. Poly(ADP-ribose) polymerase (poly(ADP[adenosinediphosphate]-ribose) polymerase, PARP) antibody, anti-ETO antibody was purchased from Santa Cruz Biotech, USA, anti-Caspase-3, Caspase-9 antibody, horseradish peroxidized The HRP-labeled secondary antibody and immunoblotting chromogenic system were purchased from Cell Signaling, USA. Actin antibody was purchased from Sigma, USA, and Cytochromec Releas...
Embodiment 3
[0112] Study on the caspase-3 cleavage site of AML1-ETO fusion protein
[0113] 1. Materials and methods
[0114] 1. Reagents and Instruments
[0115] Oridonin, fetal bovine serum see Example 1 "Materials and Methods". Caspase-3 specific inhibitor Z-DQMD-FMK see Example 2 "Materials and Methods". Anti-ETO antibody was purchased from Santa Cruz Biotech, USA. Actin antibody was purchased from Sigma, USA. Site-directed mutagenesis kit QuickChange Site-Directed Mutagenesis Kit is a product of Stratagene, USA. The Plasmid Maxi Preparation Kit is a product of QIAGEN, Germany. The Gene Pulser electroporator and the Cuvette electroporation cup are products of Bio-Rad, USA.
[0116] 2. Caspase-3 specific inhibitor Z-DQMD-FMK inhibition experiment
[0117] Kasumi-1 cell culture is the same as in Example 1. During the experiment, the cells were treated with 2-4×10 5 / ml density inoculation culture, application of 2 μ M, 5 μ M two concentrations of Rubescensine A and in the presen...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap