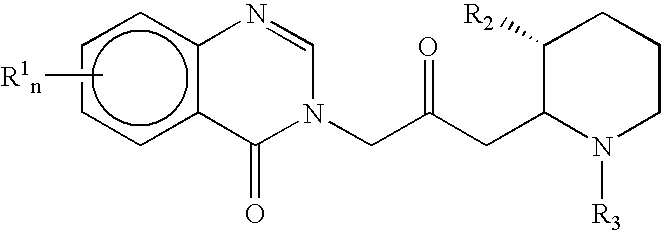
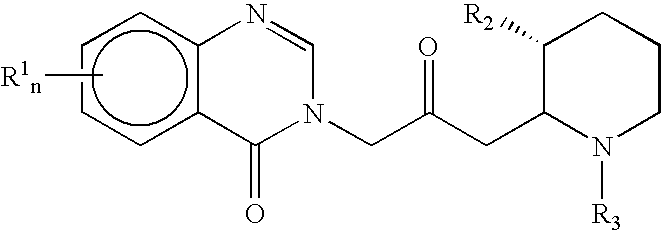
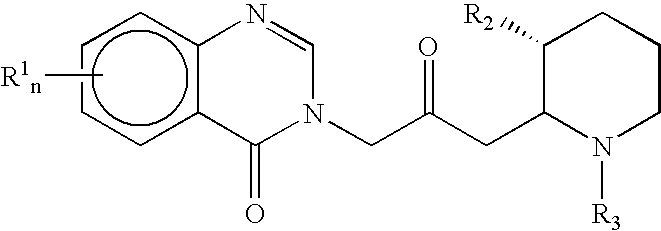
Treatment of hepatic cirrhosis
a technology for cirrhosis and hepatic fibrosis, which is applied in the field of treatment of hepatic cirrhosis, can solve the problems of concomitant decrease in liver function, inability to cure, and inability to cure,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0097] Effect of Halofuginone on Mild Fibrosis in Rat Liver
[0098] Halofuginone substantially completely prevented mild dimethylnitrosamine-induced fibrosis, as demonstrated by the measurement of collagen .alpha.1(I) gene expression and hydroxyproline content. The specific experimental method used was similar to that of Example 1, except that the dimethylnitrosamine-treated rats were only given 0.25% dimethylnitrosamine in saline, a much lower dose than that given in Example 1 above. Also, the duration of treatment was longer before the rats were sacrificed: 4 weeks as opposed to 3 weeks in Example 1.
[0099] The expression of the collagen .alpha.1(I) gene was measured as described in Example 1 above. For hydroxyproline analysis, liver samples were hydrolyzed for 22 hours at 110.degree. C. with 6 N HCl. Nitrogen was determined after Kjeldahl digestion by the spectrophotometric procedure using an autoanalyzer as described by Krom [M. D. Krom, Analyst, 105:305-16, 1980]. The collagen-uni...
example 3
[0103] Inhibition of Fibrosis Induced by Bile Duct Ligation
[0104] In addition to dimethylnitrosamine-induced liver fibrosis, a second model of liver fibrosis in rats is available. This model relies upon surgical ligation of the bile duct to induce liver fibrosis, rather than requiring the administration of exogeneous substances or toxic chemicals, and has been shown to be a suitable model for studying human liver cirrhosis [Kountaras, J. et al., Br. J. Exp. Pathol., 65:305-311, 1984; Muriel, P. et al., J. Hepatol., 21:95-102, 1994; Muriel P. et al., J. Appl. Tox., 15:449-453, 1995]. Thus, the particular advantage of the bile duct ligation model is that any protective treatments must directly protect the liver from the pathological changes induced by fibrosis, rather than indirectly altering the effects of the exogeneous substance which is used to cause liver fibrosis in the animal model. The experimental method was as follows.
[0105] Male Wistar rats, weighing 200-250 g, were divided...
example 4
[0110] Treatment of Liver Cirrhosis by Halofuginone
[0111] Another model of liver cirrhosis is the chemical induction of such cirrhosis with thioacetamide (TAA) in rats. The administration of thioacetamide by intra-peritoneal (i.p.) injection induces liver cirrhosis, including the deposition of fibrotic tissues and the loss of liver function. Halofuginone was shown to be effective for both the treatment of liver cirrhosis after the appearance of cirrhotic symptoms, and for the prevention of liver cirrhosis The latter effect is particularly surprising since previous medicaments had not been previously shown to be able to treat an existing cirrhotic condition, which is important since the administration of such medicaments to human patients is typically performed only after liver cirrhosis has arisen in the patient. The experimental method was as follows.
[0112] Male Sprague-Dawley rats were divided into three groups. Two groups were injected intraperitoneally with TAA twice weekly for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| weights | aaaaa | aaaaa |
| weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com