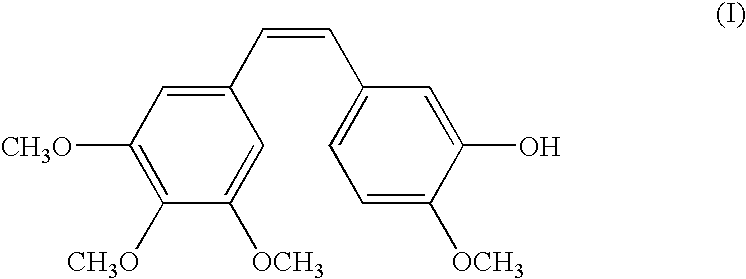
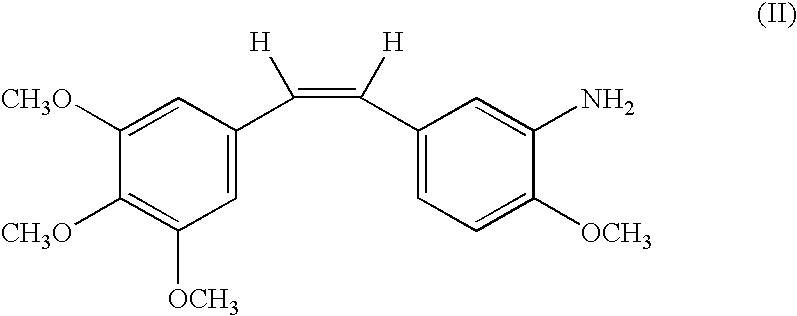
Combination comprising combretastatin and anticancer agents
a technology of combretastatin and anticancer agent, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve problems such as interfering with the reproductive cycle of cells, and achieve the effect of dissociating the toxic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In this and the following examples, RPR 258062A corresponds to the hydrochloride salt of compound IIa. The agents were administered intravenously unless otherwise specified.
IN VIVO EVALUATION OF RPR 258062AAND CISPLATINUMSched-HNTDTu-uleDoseT-CRRAgentmordaysmg / kgdaysLcKPRCRTFSSingle agents:RPR 258062AC5112,16116101.26 / 60 / 60 / 6CDDP12, 166.216.51.95 / 60 / 60 / 6Combination: -simultaneousRPR 258062A12, 16116NANA6 / 66 / 66 / 61stCDDP12, 1610- sequentialRPR 258062A1458515.95 / 55 / 50 / 5CDDP15, 1910
Abbreviations used:
HNTD = highest nontoxic dose;
T-C = tumor growth delay;
LcK = log cell kill;
RR = response rate;
PR = partial response;
CR = complete response,
TFS = tumor free survivors.
Conclusion: The combination of RPR 258062A and cisplatinum is synergistic.
example 2
IN VIVO EVALUATION OF RPR 258062A AND VINORELBINEHNTDScheduleDoseRRAgentTumordaysmg / kgT-C daysLcKPRCRTFSSingle agents:RPR 258062AMA13 / C15, 2515050.50 / 50 / 50 / 5Vinorelbine15, 2519.845.54.65 / 55 / 50 / 5Combination -sequentialRPR 258062A 1st147584.78.5*5 / 55 / 52 / 5Vinorelbine 2nd15, 2532
*log cell kill evaluated on the limited number of mice that developed tumor, the other mice in the group being tumor free survivors.
Abbreviations used:
HNTD = highest nontoxic dose;
T-C = tumor growth delay;
LcK = log cell kill;
RR = response rate;
PR = partial response;
CR = complete response,
TFS = tumor free survivors.
Conclusion: The combination of RPR 258062A and vinorelbine is synergistic.
example 3
IN VIVO EVALUATION OF RPR 258062A AND DOCETAXELScheduleHNTD DoseRRAgentTumordaysmg / kgT-C daysLcKPRCRTFSSingle agents:DocetaxelMA13 / C17, 246826.73.2*6 / 64 / 63 / 6RPR 258062A17, 2424210.81.31 / 61 / 60 / 6(2x / d)Combination -sequentialRPR 258062A16, 2315074.86.0*6 / 66 / 64 / 6(2x / d)Docetaxel17, 24109.6
*log cell kill evaluated on the limited number of mice that developed tumor, the other mice in the group being tumor free survivors.
Abbreviations used:
HNTD = highest nontoxic dose;
T-C = tumor growth delay;
LcK = log cell kill;
RR = response rate;
PR = partial response;
CR = complete response,
TFS = tumor free survivors.
Conclusion: The combination of RPR 258062A and docetaxel is synergistic.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap