Human immunodeficiency virus vaccine
a technology of immunodeficiency virus and vaccine, which is applied in the field of human immunodeficiency virus, can solve the problems of impeded efforts to develop a hiv vaccine having the desired effect, extraordinary variability of hiv, and rapid and extensive hiv mutations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Studies of Th-CTL Mutivalent in HLA B7+ Humans
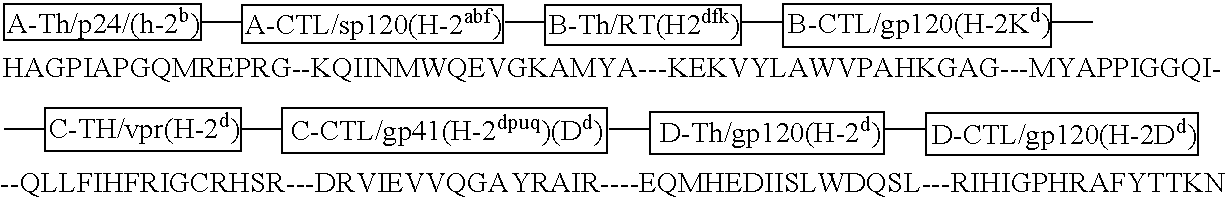
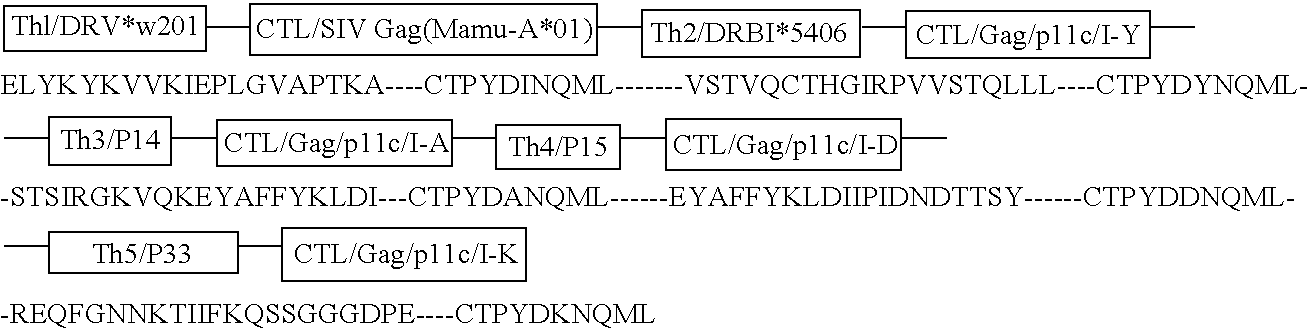
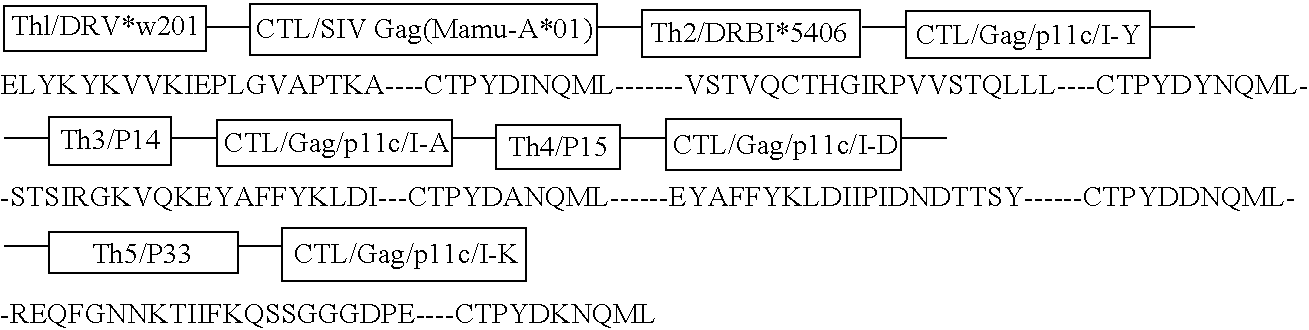
[0037] Immunogenicity and Safety of the C4-V3 Th-CTL Polyvalent Immunogen in HIV Seropositive Patients with CD4+ T Cell Counts>500 / mm3 (DATRI010). The DATR010 human trial of the C4-V3 PV immunogen has been completed (Bartlett et al, AIDS Res. Hum. Retro. 12:1291-1300 (1998)). The immunogen was 4 Th-CTL peptides with the Th epitope the same in each peptide and the CTL peptide was four variants of a B7-restricted env CTL epitope (Haynes, Res. Human Retro. 11:211-221 (1995), Beddows et al, J. Gen. Virol. 79:77-82 (1998), Table 5). Ten HIV-infected, HLA B7-positive patients with CD4+ T cells>500 / mm3 were enrolled. Eight patients received 2 mg of C4-V3 polyvalent immunogen (ie, 500 μg of each peptide) emulsified in incomplete Freund's adjuvant (Seppic ISA51) IM X5 over 24 weeks, and 2 controls received ISA51 IM alone. Vaccine recipients had excellent boosts of Th proliferative levels and neutralizing antibody levels to TCLA HIV (Bartlett et ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com