Adenovirus vectors, packaging cell lines, compositions, and methods for preparation and use
a technology of adenovirus and vector, applied in the field of gene therapy, can solve the problem of limiting the ability of ad-derived vectors to “spread” to other host cells or tissues, and achieve the effect of duplicate cell receptor binding and dna delivery properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Adenovirus Packaging Cell Lines
[0259]Cell lines that are commonly used for growing adenovirus are useful as host cells for the preparation of adenovirus packaging cell lines. Preferred cells include 293 cells, an adenovirus-transformed human embryonic kidney cell line obtained from the ATCC, having Accession Number CRL 1573; HeLa, a human epithelial carcinoma cell line (ATCC Accession Number CCL-2); A549, a human lung carcinoma cell line (ATCC Accession Number CCL 1889); and the like epithelial-derived cell lines. As a result of the adenovirus transformation, the 293 cells contain the E1 early region regulatory gene. All cells were maintained in complete DMEM+10% fetal calf serum unless otherwise noted.
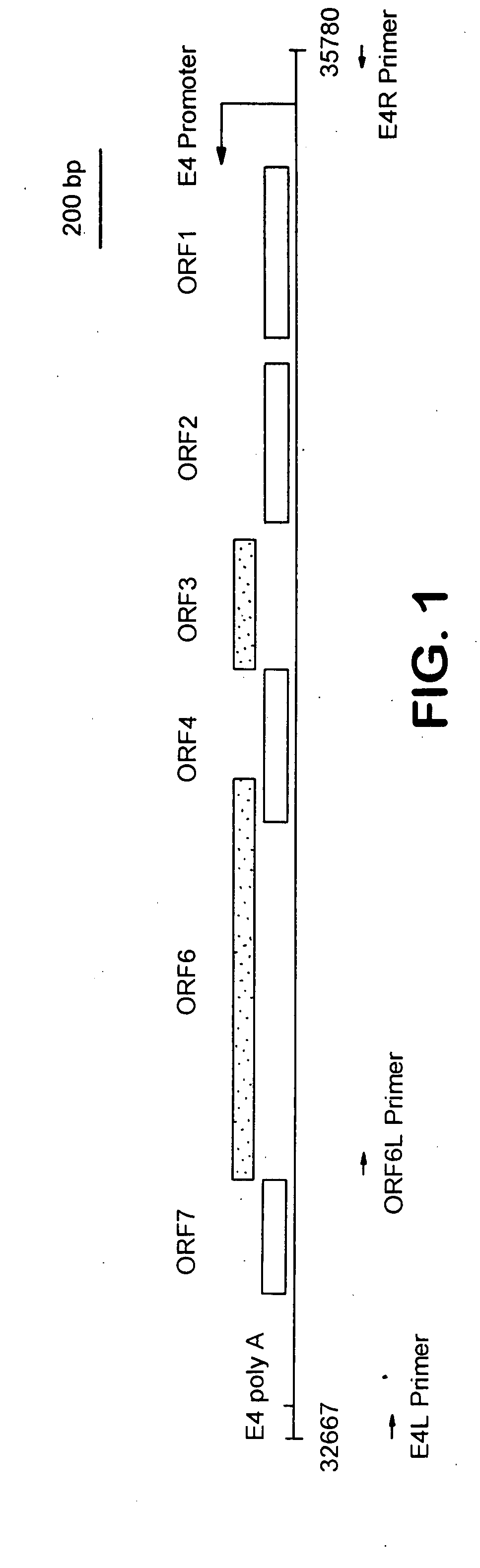
[0260]The cell lines of this invention allow for the production and propagation of novel adenovirus-based gene delivery vectors having deletions in preselected gene regions, that are obtained by cellular complementation of adenoviral genes. To provide the desired comple...
example 2
Preparation of Adenoviral Gene Delivery Vectors Using Adenoviral Packaging Cell Lines
[0298]Adenoviral delivery vectors of this invention are prepared to separately lack the combinations of E1 / fiber and E4 / fiber. Such vectors are more replication-defective than those previously in use due to the absence of multiple viral genes. A preferred adenoviral delivery vector of this invention that is replication competent but only via a non-fiber means is one that only lacks the fiber gene but contains the remaining functional adenoviral regulatory and structural genes. Furthermore, the adenovirus delivery vectors of this invention have a higher capacity for insertion of foreign DNA.
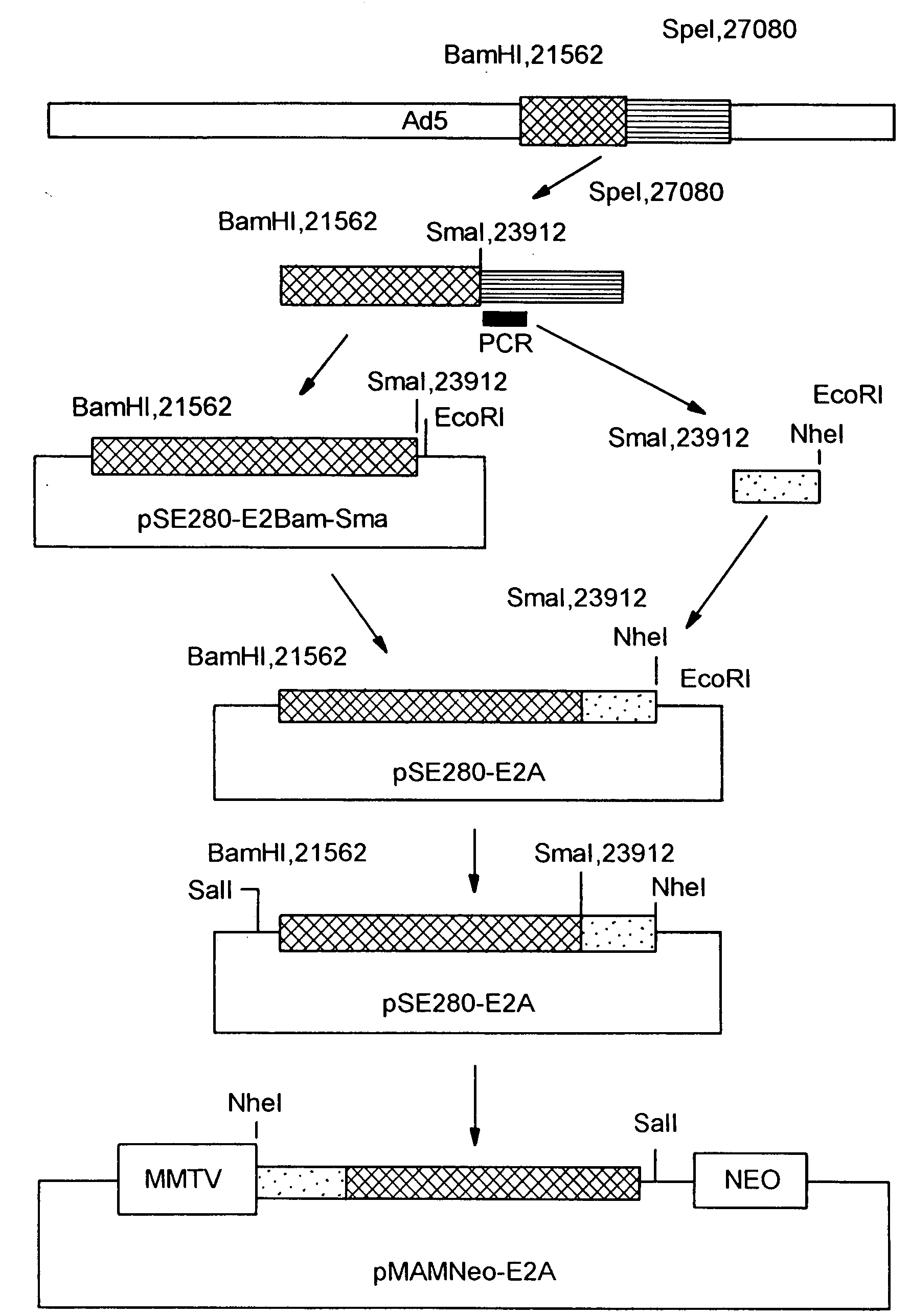
[0299]A. Preparation of Adenoviral Gene Delivery Vectors Having Specific Gene Deletions and Methods of Use
[0300]To construct the E1 / fiber deleted viral vector containing the LacZ reporter gene construct, two new plasmids were constructed. The plasmid pΔE1Bβgal was constructed as follows. A DNA fragment containing ...
example 3
Deposit of Materials
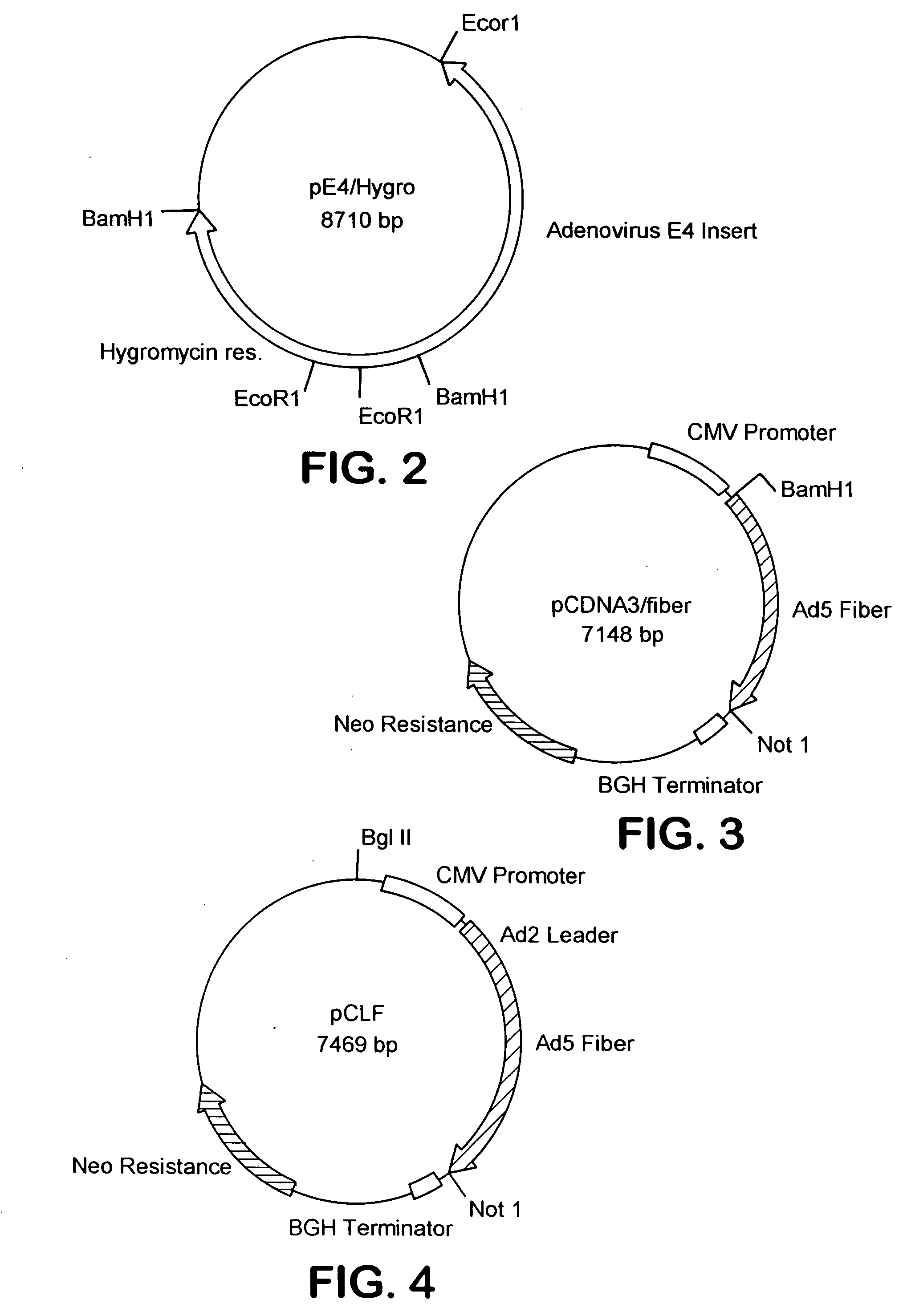
[0347]The following cell lines and plasmids have been deposited on Sep. 25, 1996, with the American Type Culture Collection, 10801 University Blvd, Manassas, Va., USA (ATCC) under the provisions of the Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purpose of Patent Procedure and the Regulations thereunder (Budapest Treaty):Plasmid pE4 / Hygro (accession number 97739), Plasmid pCLF (accession number 97737), 211 Cell Line (accession number CRL-12193) and 211A Cell Line (accession number CRL-12194).
[0348]The following virus, Ad5.βgal.ΔF, was deposited on Jan. 15, 1999, with the ATCC as listed above and provided with accession number VR2636.
[0349]Additionally, plasmids pDV60, pDV67, pDV69, pDV80 and pDV90 were deposited at the ATCC on Jan. 5, 2000 and provided with accession numbers PTA-1144, PTA-1145, PTA-1146, PTA-1147 and PTA-1148 respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com