In vivo ctl elicitation by heat shock protein fusion proteins maps to a discrete domain and is cd4+ t cell-independent
a heat shock protein and fusion protein technology, applied in the field of in vivo ctl elicitation by heat shock protein fusion proteins, can solve the problems of inability to stimulate the production of cd8 t cells, generally fail to elicit effective cd8 t cell responses, etc., to achieve high-effective cd8+ ctl responses, prevent or reduce the length or severity of symptoms, and facilitate treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
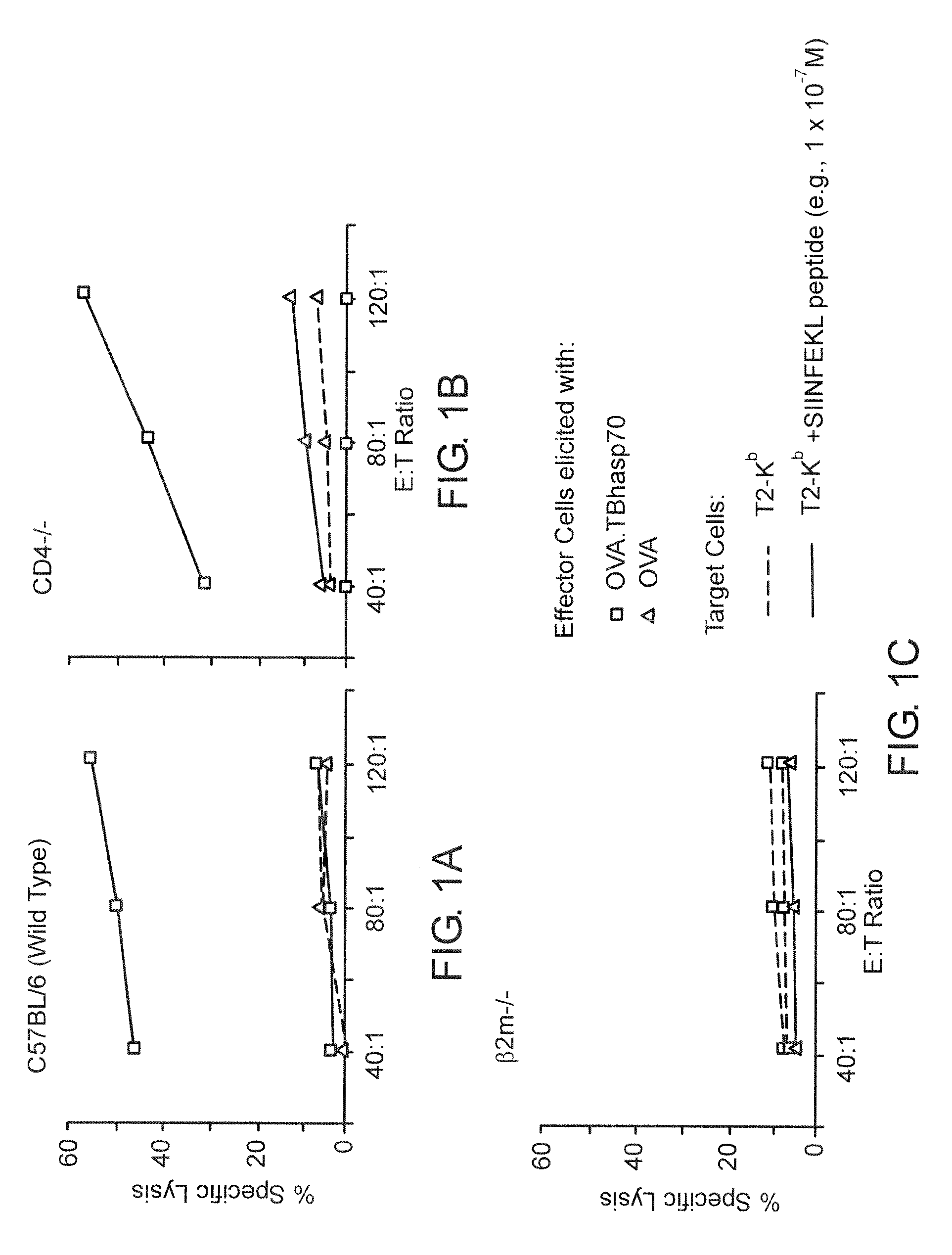
In Vivo CTL Elicitation by hsp70 Fusion Proteins Maps to a Discrete Domain and is CD4+ T Cell-Independent
Materials and Methods
Expression Vectors:
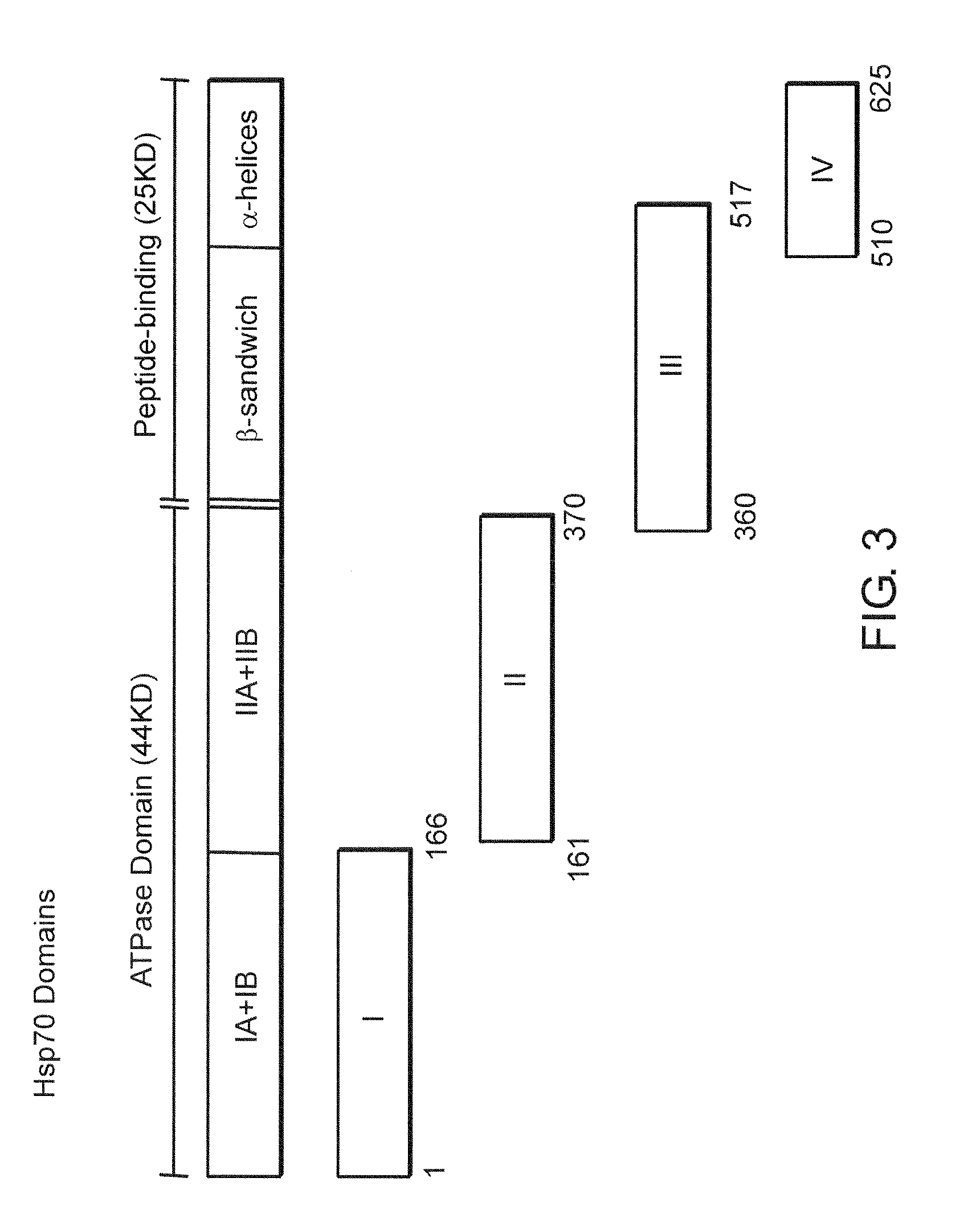
[0051]All constructs used to produce OVA.hsp70 fusion proteins were made in the bacterial expression plasmid pKS11h (Suzue, K., et al., Proc. Natl. Acad. Sci. USA, 94:13146-13151 (1997)). Fusion constructs, consisting of OVA fused to the N-terminus of various segments of hsp70, were inserted downstream of the histidine tag sequence. A portion of ovalbumin (amino acid 230-359, hereafter referred to as OVA) was amplified from pOV230 (McReynolds, L. A., et al. Gene, 2:217-231 (1.977)) by PCR using upstream primer oQH025 and the downstream primer oQH027. Functional and structural domains of TBhsp70 based on crystal structures of ATP domain of bovine hsc70 (Flaherty, K. M., et al., Nature, 346:623-628 (1990)) and peptide-binding domain of E. coli DnaK (Zhu, X., et al., Science, 272:1606-1614 (1996)) were used. The lull-length TBhsp70 were separa...
example 2
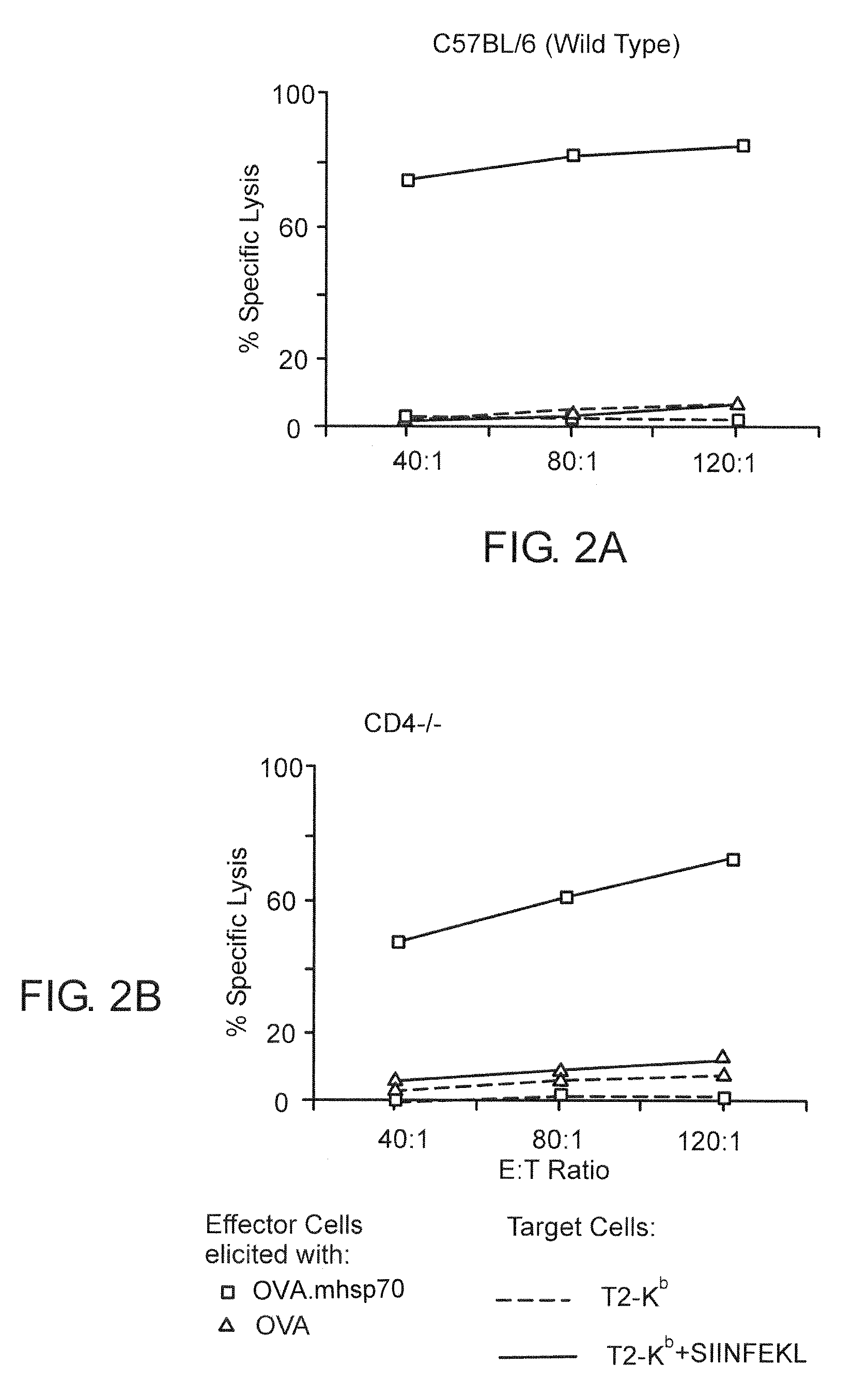
Heat Shock Fusion Proteins Stimulate Dendritic Cells and Elicit
[0065]Production of Cytolytic T Lymphocytes Without Requiring Participation of CD4 T Cells
Methods and Materials
Mice, CTL Clones and Cell Lines
[0066]C57BL / 6 (H-2b), Cd-4-deficient (CD4 tm1Mak, H-2b), and C3H / HeJ mice (H-2k) were obtained from The Jackson Laboratories (Bar Harbor, Me.), maintained in barrier cages under specific pathogen free conditions, and immunized between 4- and 10-weeks of age. 2C TCR transgenic mice (H-2b) contain the rearranged transgenes encoding the ÿÿ TCR from a 2C CTL clone (Sha, W., et al., Nature, 335:271-274 (1988)), 2C TCR transgenic mice deficient for the recombination activating gene-1 (termed 2C / RAG) (Manning, T., et al., J. Immunol., 159:4665-4675 (1997)) were used as a source of naive T cells for in vitro assays (Cho, B., et al., Proc. Natl. Acad. Sci. USA, 96:2976-2981 (1999)), 2C CTL clone, L3.100, has been previously described (Sykulev, Y., et al., Immunity, 9:475-483 (1998)), EL4 ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com