Methods of treatment of endobronchial infections
a treatment method and endobronchial technology, applied in the direction of antibacterial agents, aerosol delivery, metabolic disorders, etc., can solve the problems of high polarity of parenteral aminoglycosides, poor penetration of endobronchial space, high cost and inconvenience, and create a potential for significant treatment burden for persons with
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Tobramycin Powder for Inhalation (TPI)
[0062]A tobramycin sulfate dry powder composition is prepared according to the following procedure. Sterile Water for Irrigation (SWFI) is heated above the gel to liquid crystal temperature (about 80° C.) of disteroyl phosphatidylcholine (DSPC). DSPC and calcium chloride dihydrate are then added to the heated water. The resulting lipid dispersion is mixed in an UltraTurrax T-50 (IKA Labortechnik) at 8,000 rpm for 5 min. Perfluorooctyl bromide (PFOB) is then added dropwise (15 ml / min) to the lipid dispersion under mixing. After the addition is complete the resulting PFOB-in-water emulsion is mixed for an additional 10 min at 10,000 rpm. Emulsification in the UltraTurrax produces droplets in the micron-size range. Tobramycin sulfate is then dissolved in the continuous phase of the emulsion and the resulting dispersion is used as the feedstock for spray drying. The feedstock is then spray dried to obtain a dry powder formulation havi...
example 2
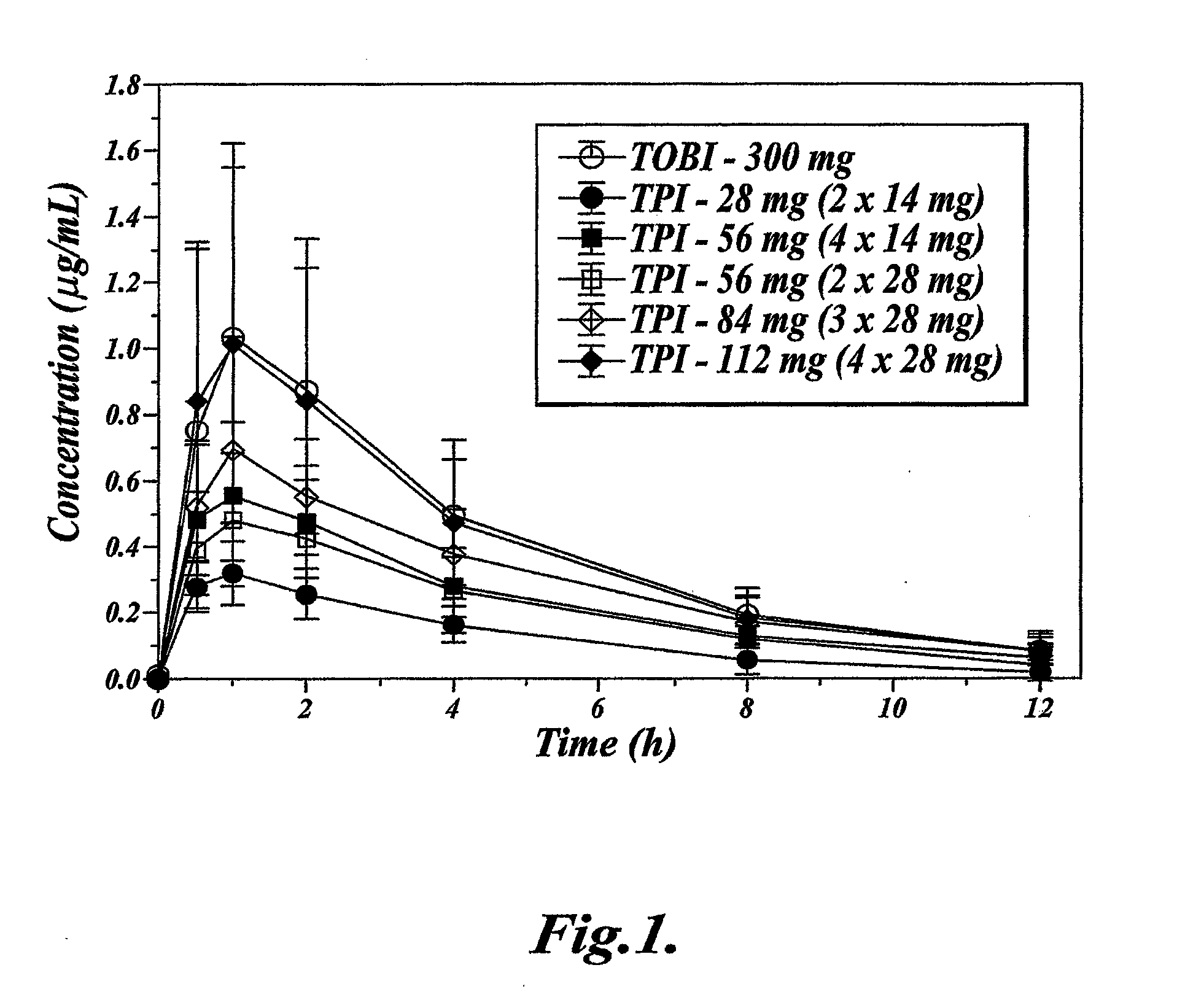
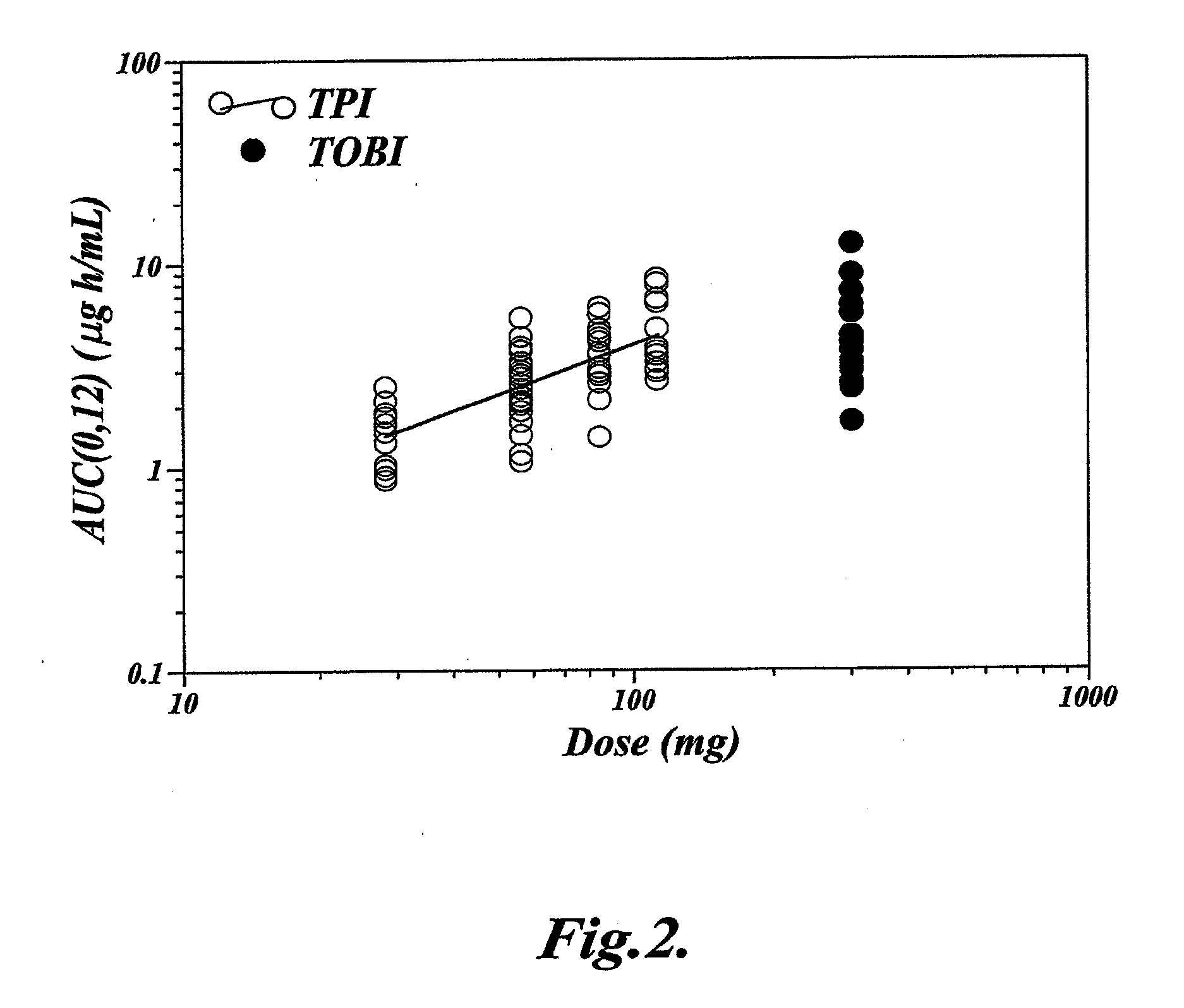
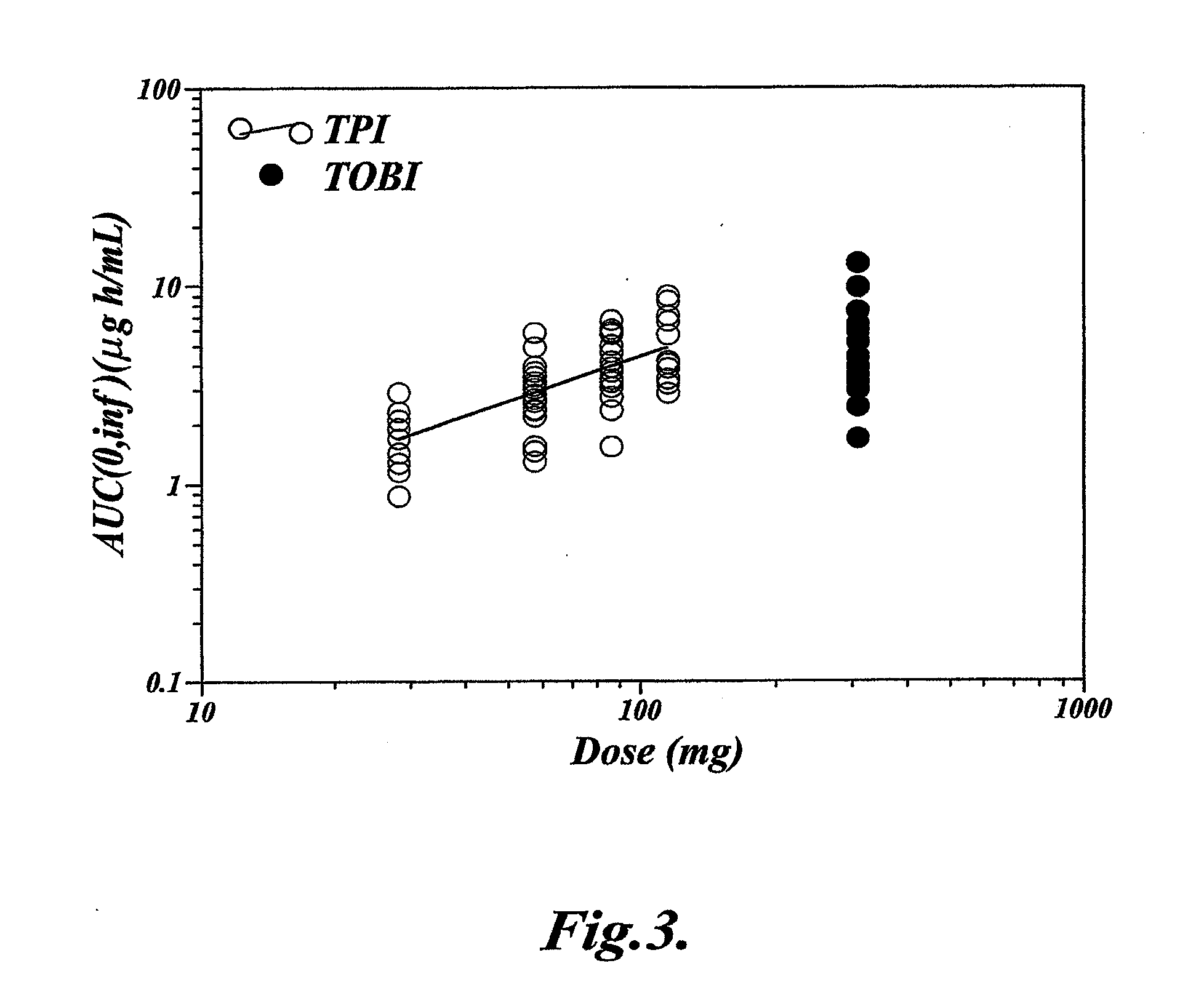
[0064]This Example describes a clinical study that demonstrates that single dose administration of a tobramycin dry powder composition of the invention results in a more efficient delivery of tobramycin than administration of a tobramycin solution, while maintaining similar tobramycin pharmacokinetics.
[0065]Overall Study Design and Plan
[0066]The study was designed as a randomized, open-label, sequential-cohort, active-controlled, single-dose, dose-escalation study. In each sequential cohort, subjects were randomized in a 3:1 ratio to receive either a single dose of Tobramycin Powder for Inhalation (TPI) administered using a T-326 Inhaler (Nektar Therapeutics, San Carlos, Calif., USA), according to the dosing schedule shown below, or a single dose of 300 mg Tobramycin Solution for Inhalation (TOBI), aerosolized by a PARI LC PLUS™ jet nebulizer with a DeVilbiss PulmoAide™ compressor. Subjects were allowed to participate in one cohort only.
[0067]Escalation to the next TPI treatment coh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aerodynamic diameter | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com