Fractionated products obtained from gamboge resin, and medical uses of the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Analysis of an Acetone-Extracted Product from Gamboge Resin (TSB-14) by Analytical RP-HPLC
[0169]An acetone-extracted product from gamboge resin, i.e., TSB-14, which was prepared according to Example 1 of U.S. Pat. No. 7,138,428 B2, was subjected to an analytical RP-HPLC analysis, so as to determine the chemical constituent(s) contained therein.
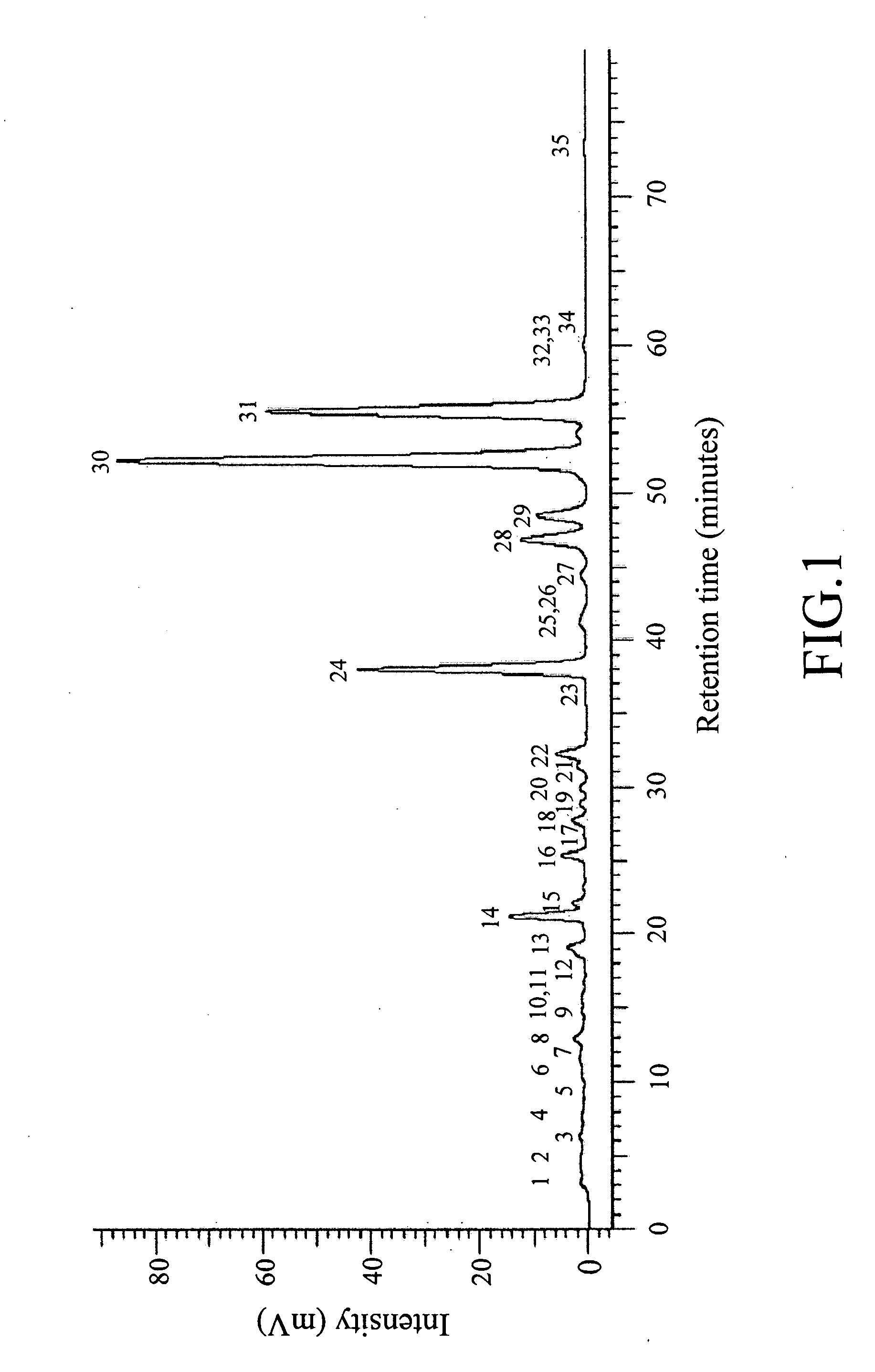
[0170]Briefly, 1.0 mg of the product TSB-14 was dissolved in 1 mL of acetone and then subjected to an analytical RP-HPLC analysis along the lines as described in the previous section of “General Procedures,” and an elution profile as shown in FIG. 1 was obtained.
[0171]Referring to FIG. 1, 35 major peaks (numbered from 1 to 35) are present in the elution profile of the product TSB-14 within the retention time of 0 to 80 minutes. According to the results shown in FIG. 1, the applicants postulated that it might be possible to isolate 35 compounds from the product TSB-14. To verify this postulation, the product TSB-14 was subjected to the followin...
example 2
Preparation of Purified Compounds from the Product TSB-14
A. Semi-Preparative RP-HPLC Analysis of the Product TSB-14
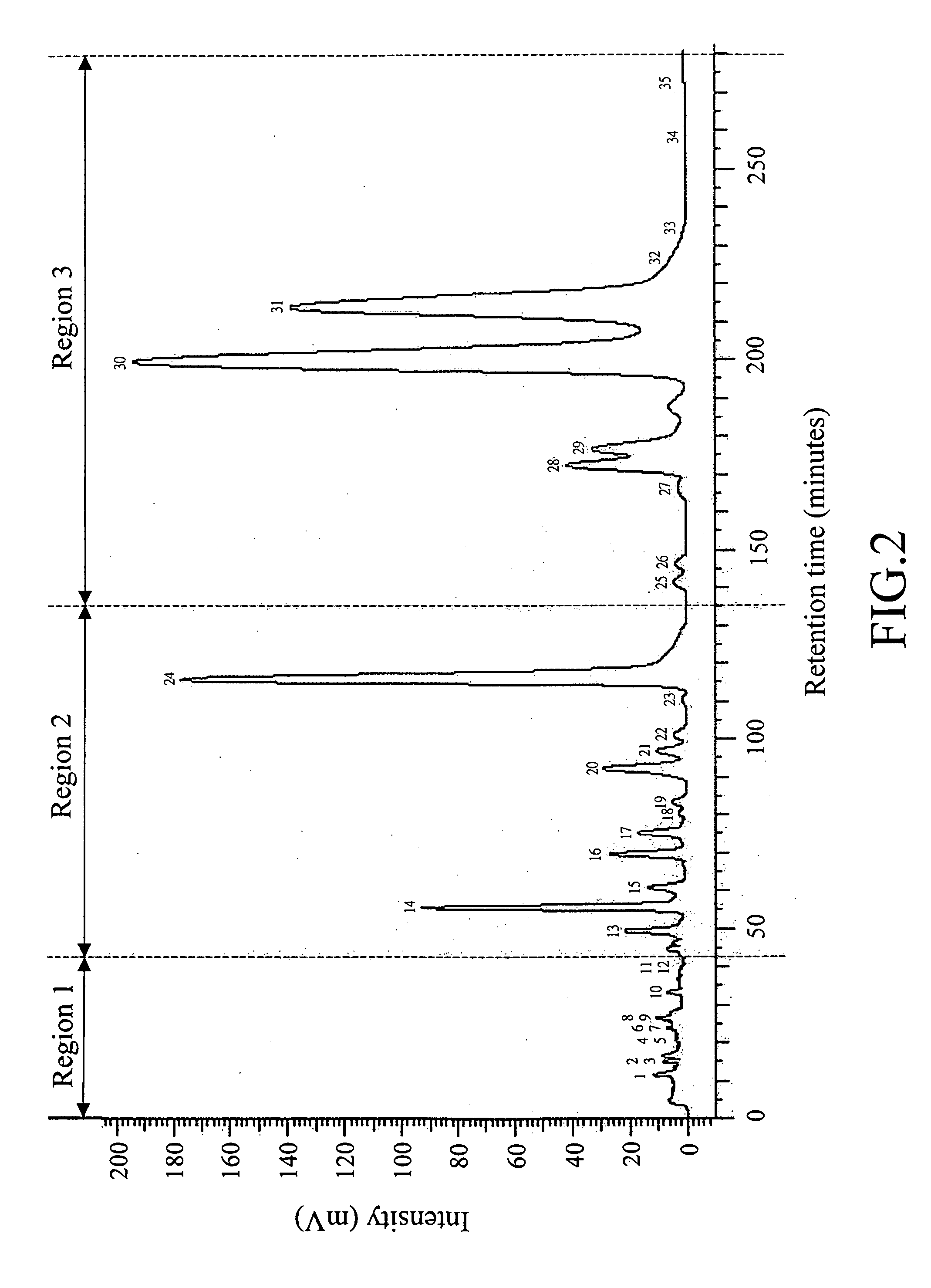
[0172]3 g of the product TSB-14 was dissolved in 30 mL of acetone / acetonitrile (v / v=1:9) and then subjected to a semi-preparative RP-HPLC analysis along the lines as described in the previous section of “General Procedures,” in which a mobile phase containing 0.05% TFA(aq.) / 65% acetonitrile(aq.)(35:65) was used. The elution profile thus obtained is shown in FIG. 2.
[0173]After comparing the two elution profiles shown in FIGS. 1 and 2, the major peaks 1-35 as identified in FIG. 1 were also identified in the elution profile of FIG. 2, which was subsequently divided into three regions, i.e., Region 1, which includes peaks 1-12 eluted at a retention time of 0 to 42 minutes; Region 2, which includes peaks 13 to 24 eluted at a retention time of 42 to 135 minutes; and Region 3, which includes peaks 25 to 35 eluted at a retention time of 135 to 280 minutes.
B. Preparation of Frac...
example 3
Identification and Characterization of Compounds Isolated and Purified from Fractions 1-3
[0188]The physical and chemical properties of the thirty five products purified as obtained from fractions 1-3 in the above Example 2 were analyzed according to the methodologies set forth in the preceding section of “General Procedures,” including melting point (mp) measurement, 1H-NMR, 13C-NMR, 1H-1H COSY, HMQC, HMBC, NOESY, EIMS, HREIMS, FABMS, and HRFABMS. The experimental data thus obtained are summarized below.
1. Product Gh-47:
[0189]Product Gh-47, which was purified from eluate 13 of fraction 2, was determined to have the following properties:
[0190]Yellow flake crystals; mp 204˜209° C.
[0191]EIMS m / z (relative intensity): 560 [M]+ (100), 545 (47), 532 (22), 517 (36), 405 (44), 389 (11), 363 (24), 349 (17), 307 (12), 287 (22), 285 (16), 245 (15), 215 (12), 189 (5).
[0192]1H-NMR (400 MHz, CDCl3): δ 7.56 (1H, d, J=6.9 Hz), 6.63 (1H, d, J=9.6 Hz), 5.51 (1H, d, J=9.6 Hz), 5.12 (1H, dd, J=13.6, 6....
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap