Treatment Of HIV-1 Infection And AIDS
a technology for treating hiv-1 infection and aids, which is applied in the direction of drug compositions, enzyme inhibitors, peptide/protein ingredients, etc., can solve the problems of reducing the number of t-cells, affecting the survival rate of patients, so as to reduce inflammation and slow the progression of diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General
[0349]i. Culture and Infection of HLACs
[0350]Human tonsil or splenic tissues from routine tonsillectomies were obtained from the National Disease Research Interchange and the Cooperative Human Tissue Network and processed as previously described (Jekle et al., 2003, J Virol 77:5846-5854). In brief, tonsils or spleen were minced, passed through a 40-μm cell strainer, and cultured in 96-well U-bottomed polystyrene plates (2×106 cells / well) in medium (200 μl / well) consisting of RPMI 1640 supplemented with 15% heat-inactivated fetal bovine serum, 100 μg / ml gentamicin, 200 μg / ml ampicillin, 1 mM sodium pyruvate, 1% nonessential amino acids (Mediatech), 2 mM L-glutamine, and 1% fungizone (Invitrogen). All HIV-1 infections were carried out with 20-50 ng of HIV-1 p24gag. Cells were incubated with the virus for 12-16 h, washed extensively, and supplemented with fresh medium. After day 5, infections were monitored by measuring p24gag levels in the culture medium using a FLAQ assay (Hay...
example 2
Selective Depletion of CD4 T-Cells by X4-Tropic HIV-1
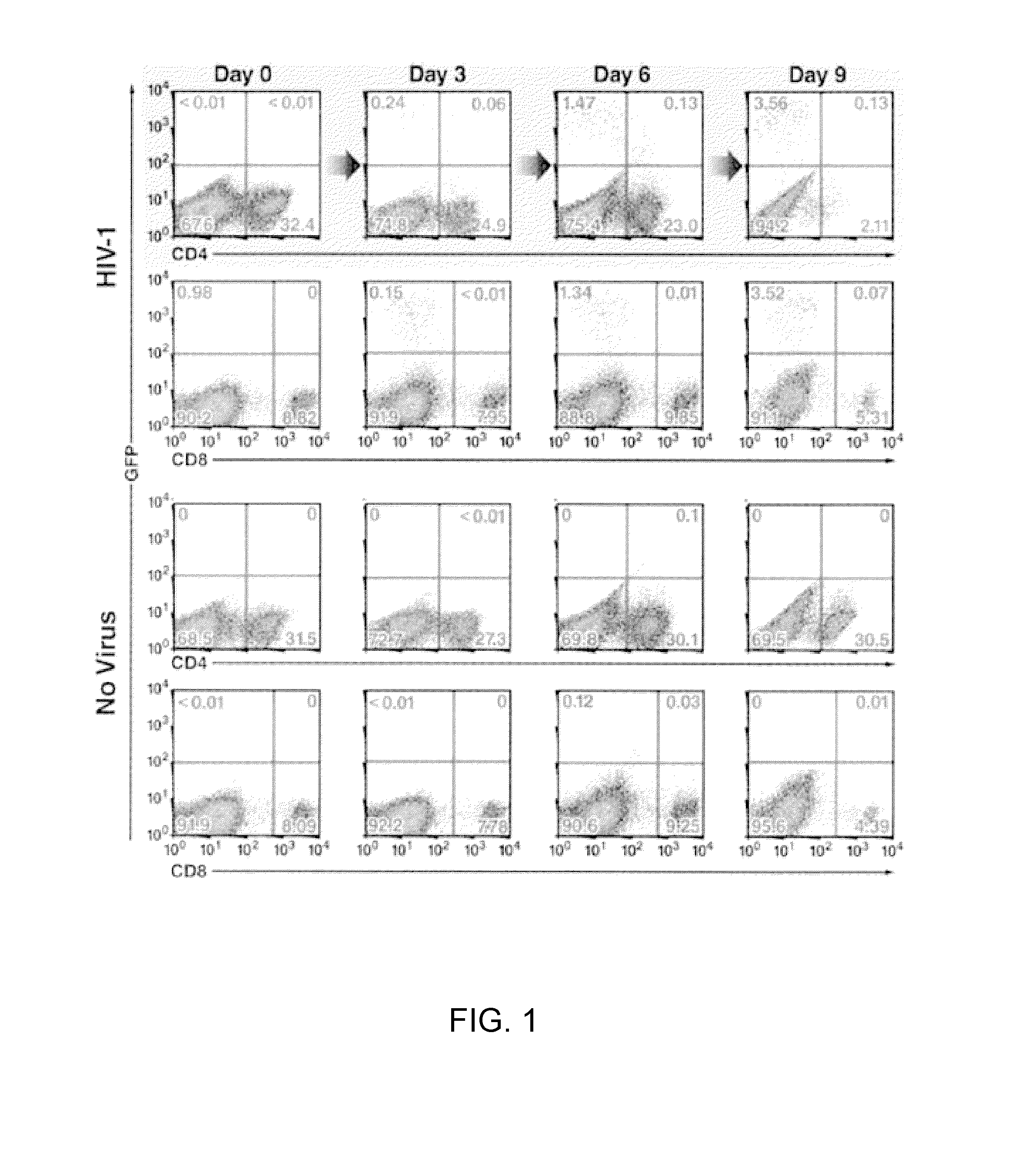
[0386]To explore depletion of CD4 T-cells by HIV-1, HLACs made from freshly dissected human tonsillar tissues were infected with a GFP reporter virus (NLENG1), prepared from the X4-tropic NL4-3 strain of HIV-1. This reporter produces fully replication-competent viruses. An IRES inserted upstream of the Nef gene preserves Nef expression and supports LTR-driven GFP expression (Levy et al., 2004, Proc Natl Acad Sci USA 101:4204-4209), allowing simultaneous quantification of the dynamics of HIV-1 infection and T-cell depletion. NL4-3 was selected because tonsillar tissue contains a high percentage of CD4 T-cells expressing CXCR4 (90-100%). Productively infected GFP-positive cells appeared in small numbers 3 days after infection, peaked on days 6-9, and decreased until day 12, when few CD4 T-cells remained in the culture (FIG. 1) Fluorescence-linked antigen quantification (FLAQ) assay of HIV-1 p24 (Hayden et al., 2003, AIDS 17:629-631)...
example 3
Extensive Depletion of Non-Productively Infected CD4 T-Cells in HLACs
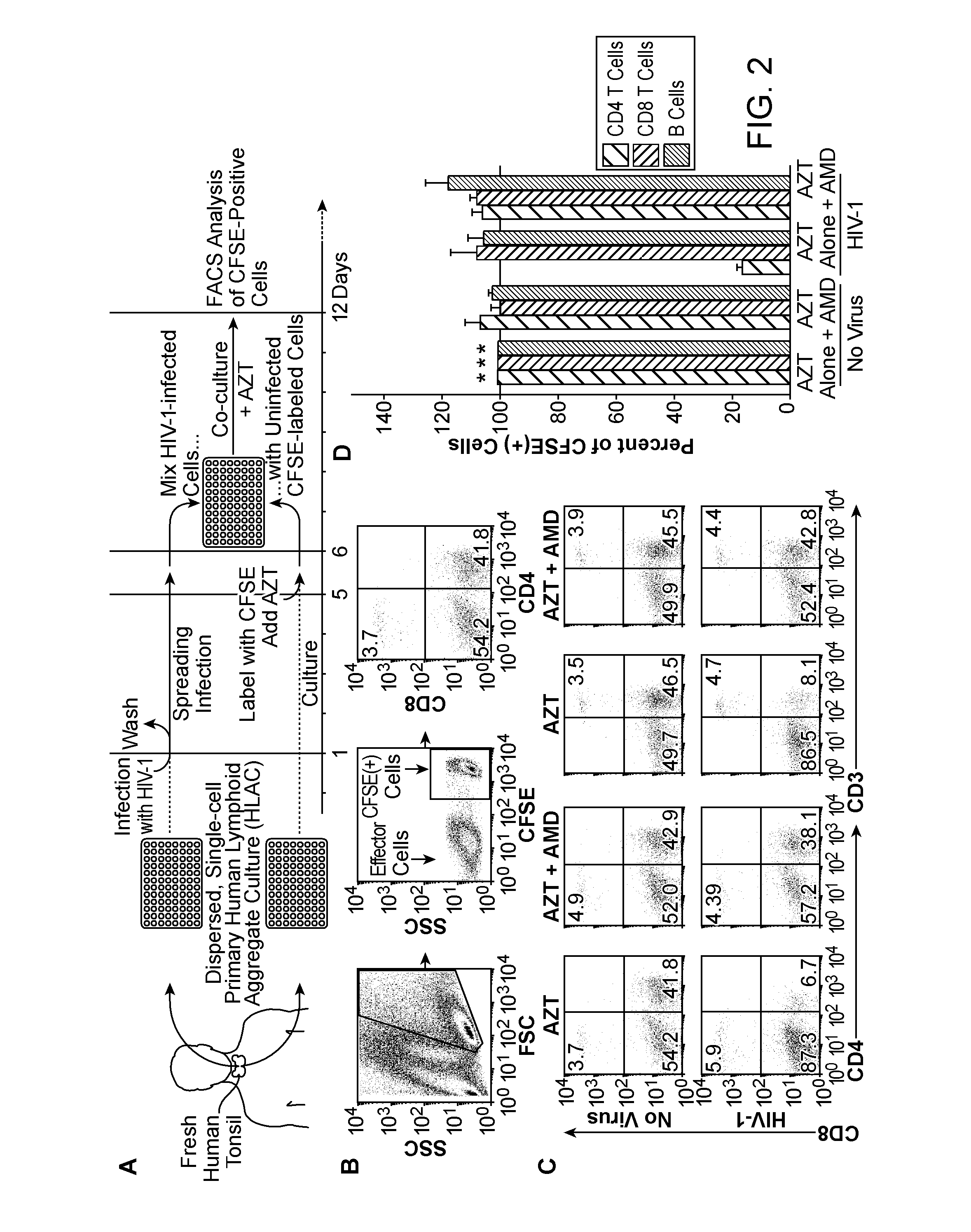
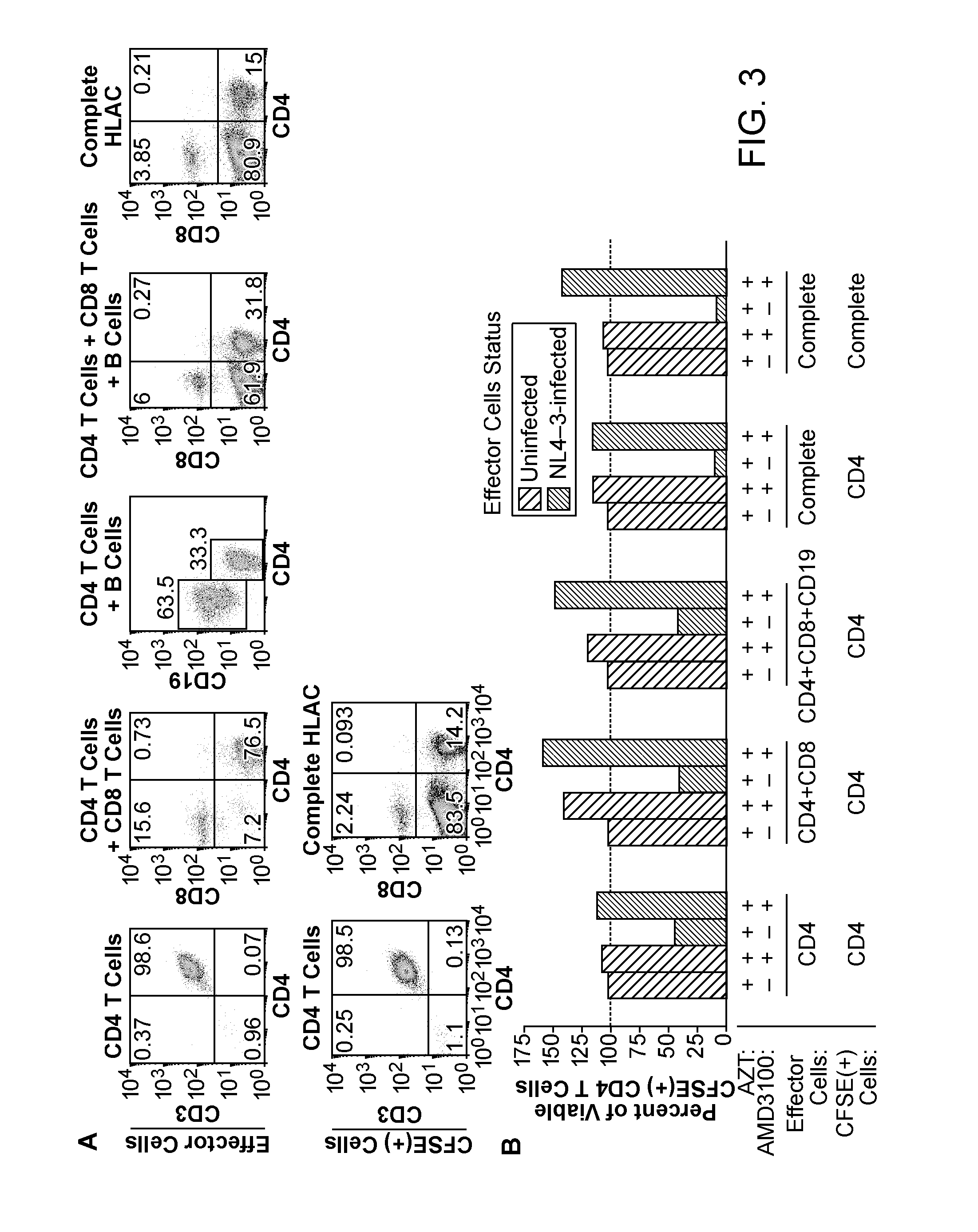
[0387]To determine if indirect killing (formerly indicated as “bystander”) of CD4 T-cells accounted for most of the observed cellular depletion, an experimental strategy (Jekle et al., 2003, J Virol 77:5846-5854) was employed that unambiguously distinguishes between the death of productively and non-productively infected cells (FIG. 2A). After 6 days of co-culture, survival analysis of CFSE-labeled cells by flow cytometry (FIG. 2B) showed extensive depletion of CD4 T-cells in cultures mixed with HIV-infected cells but not in those mixed with uninfected cells (FIG. 2C). The relative proportion of CD8 T-cells was not altered. CD3+ / CD8T-cells were similarly depleted, indicating that the loss was not an artifact of downregulated surface expression of CD4 following direct infection. Loss of CFSE-labeled CD4 T-cells was prevented by AMD3100, which blocks the engagement of gp120 with CXCR4, but not by the reverse transcri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com