Pharmaceutical compositions for combination therapy
a combination therapy and pharmaceutical composition technology, applied in drug compositions, metabolic disorders, cardiovascular disorders, etc., can solve the problems of severe side effects, impaired motor function, depression, etc., to improve the therapeutically beneficial effects of tetrabenazine, reduce adverse motor and affective effects, and alleviate involuntary movements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0181]The invention is further illustrated with reference to the following examples, which are not intended to be in any way limiting to the scope of the invention as claimed.
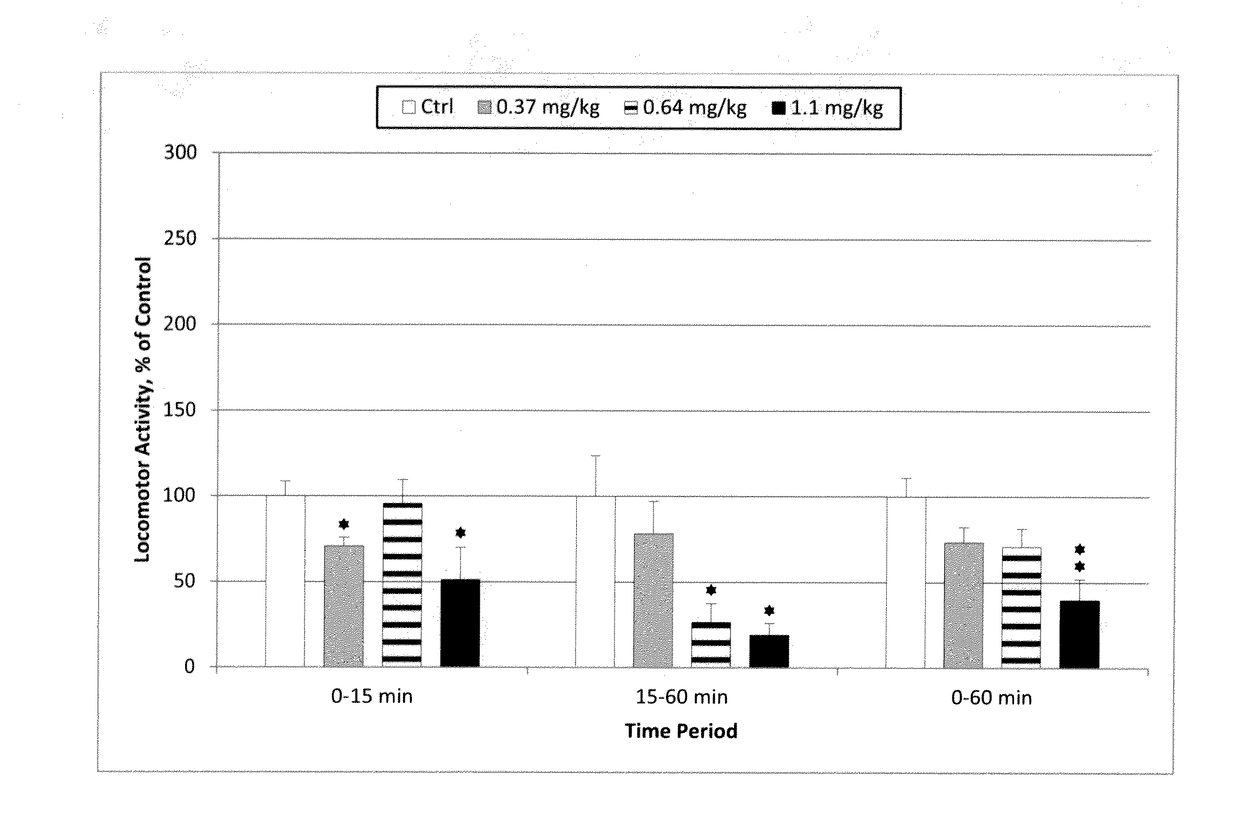
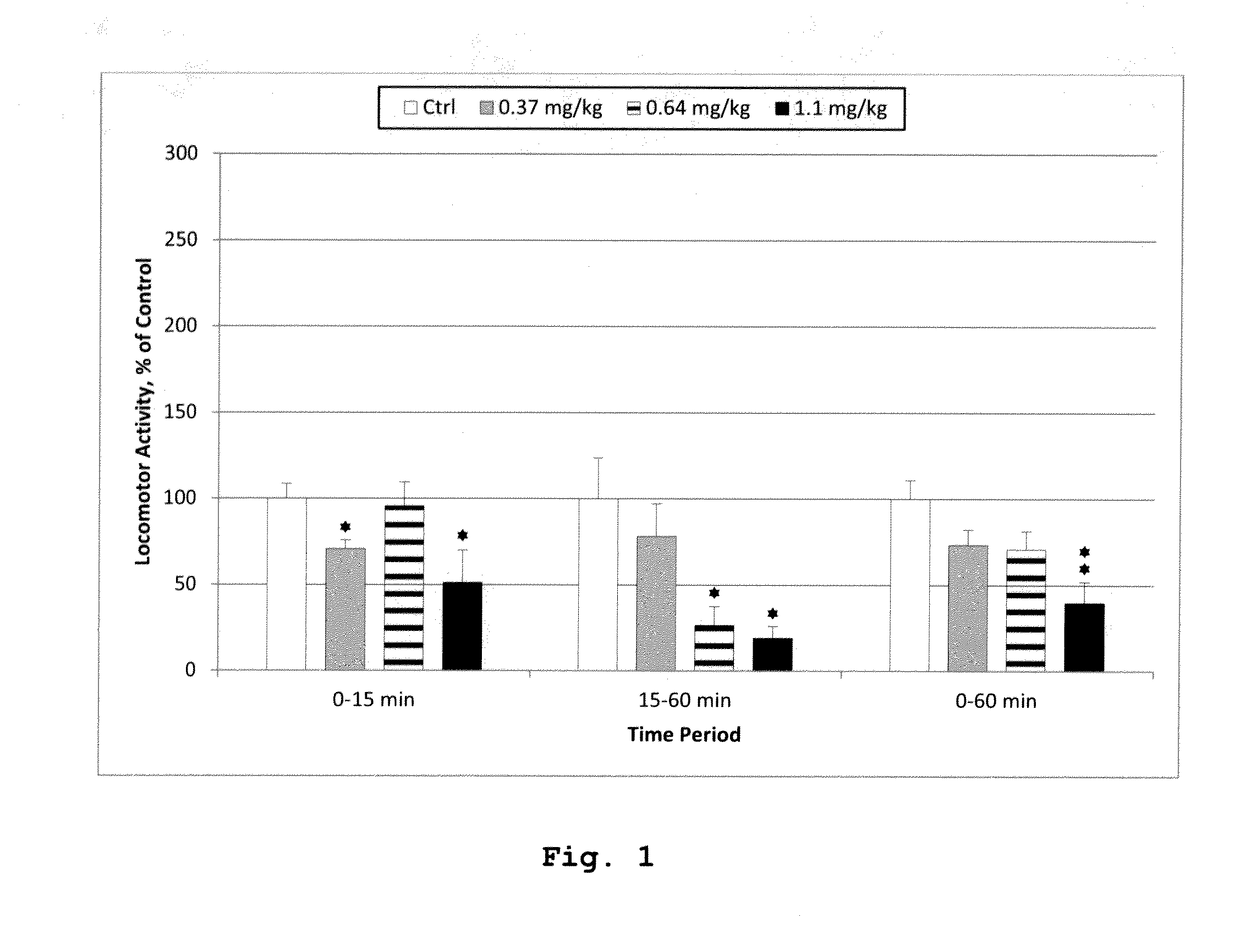
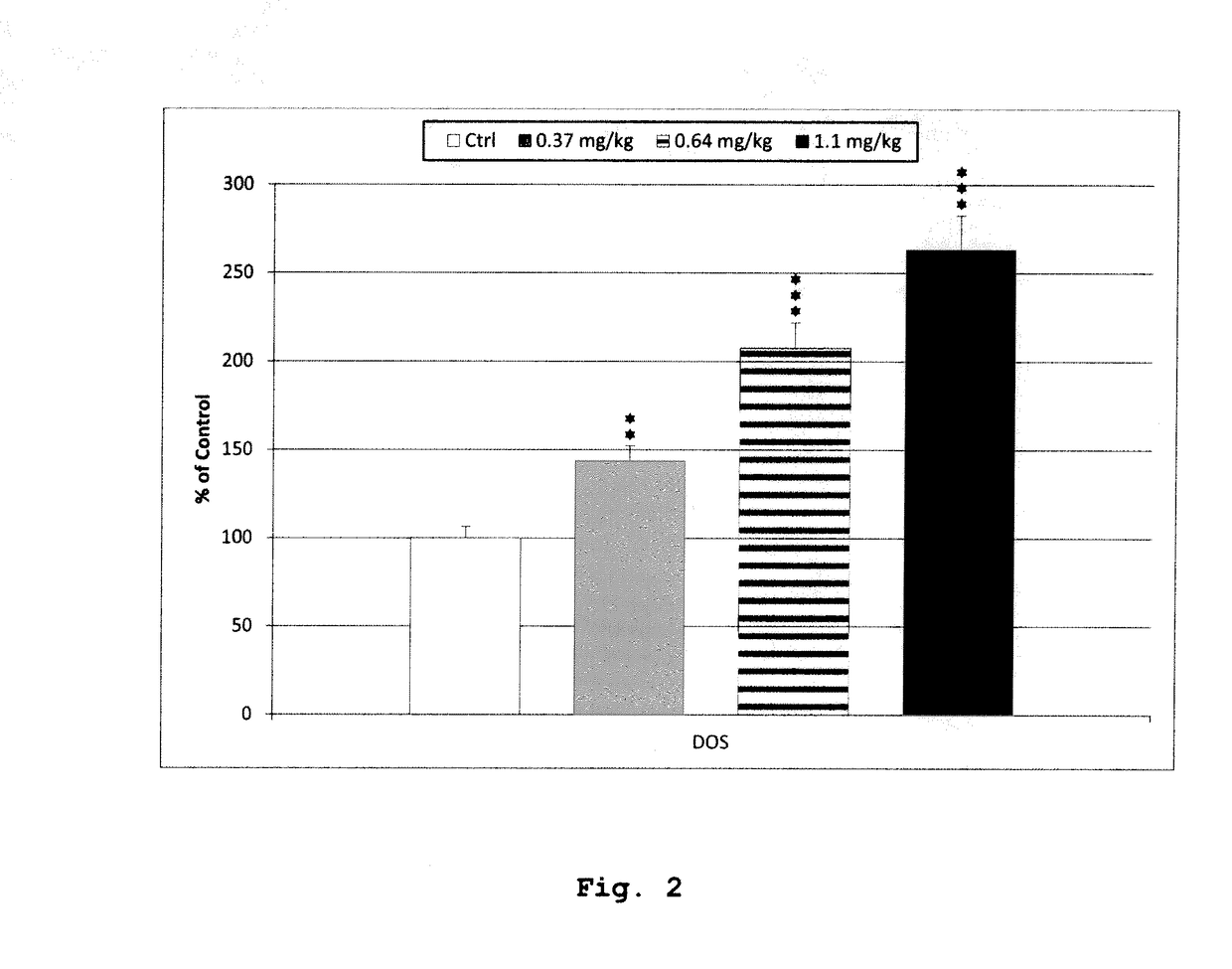
[0182]The examples below explore the interaction between Pridopidine and Tetrabenazine with respect to locomotor activity. Striatal levels of dopamine and DOPAC were also determined. Tetrabenazine reduces tissue levels of dopamine as a direct consequence of the inhibition of VMAT. Both compounds increase striatal DOPAC levels in a dose-dependent manner in vivo, reflecting decreased tone at the dopamine D2 receptor (Ponten 2010; Reches, 1983). Furthermore, the effect on expression of the immediate-early gene Arc (activity-regulated cytoskeleton-associated protein / activity-regulated gene 3.1) was measured in the frontal cortex and striatum. Arc gene expression is a biomarker reflecting synaptic activity (Steward, 2001; Kawashima 2009). Interaction experiments with Tetrabenazine and the dopamine D2 antagonist halo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com