Light therapy system
a light therapy and system technology, applied in light therapy, radiation therapy, therapy, etc., can solve the problems of voiding the warranty immediately, affecting clinical performance, and grt solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
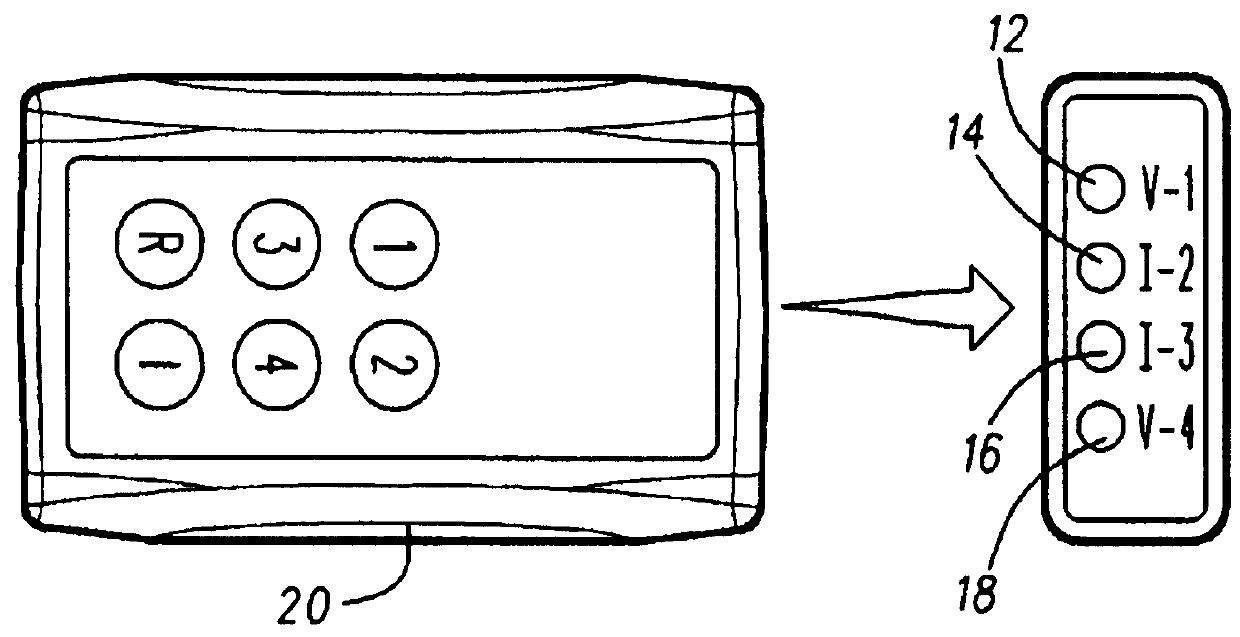
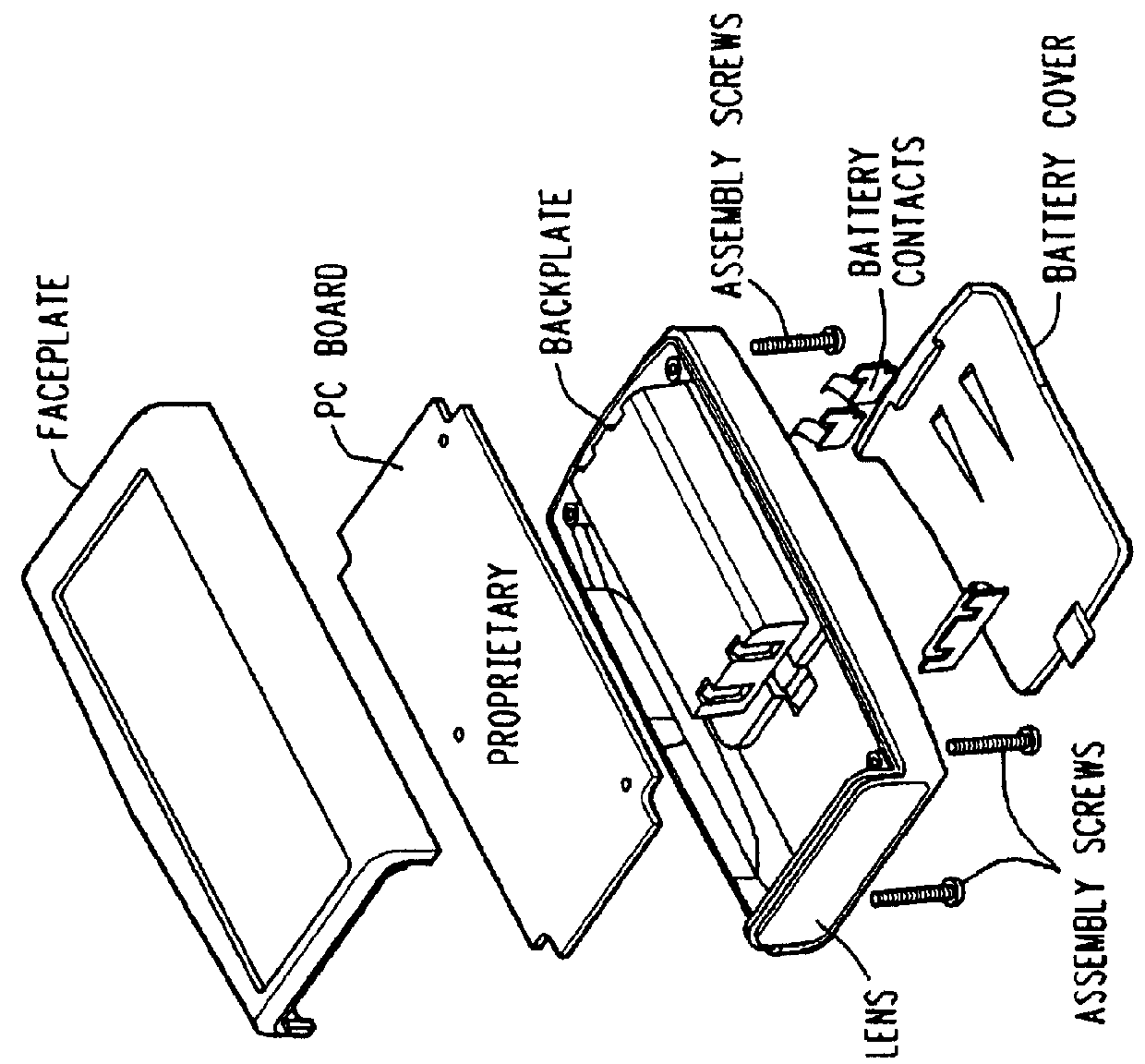
[0013]The Model PRO-8A is a hand-held, non-invasive, pain therapy system which utilizes four nonheating light emitting diodes (LED) consisting of two visible LED's and two infrared LED's in one system, It combines the clinically accepted therapeutic treatment of numerous predicate light therapy systems currently in commercial distribution and 510(k) approved.
[0014]The system consists of a basic hand-held, battery operated, control unit with the four LED's emitting light through a special red acrylic lens which does not absorb any light transmission. The visible LEDs operate at a measured wavelength of 628 nm (±5%) and the infrared LEDs operate at a measured wavelength of 850 nm (±5%), The Model PRO-8A complies with all performance, labeling, and manufacturing standards set forth in 21 CFR.
[0015]The GRT LITE Model PRO-8A and the aforementioned predicate devices emit visible and invisible photonic energy to human tissue. The comparing of the technologies is dependent on the laws of ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com