Doxazosin mesilate slow releasing preparation
A technology of doxazosin mesylate and sustained-release preparation, applied in the field of medicine, can solve the problems of patient health threat, inconvenience to patients and doctors, etc., and achieve the effects of reducing the number of doses, being convenient to carry, and reducing the incidence of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
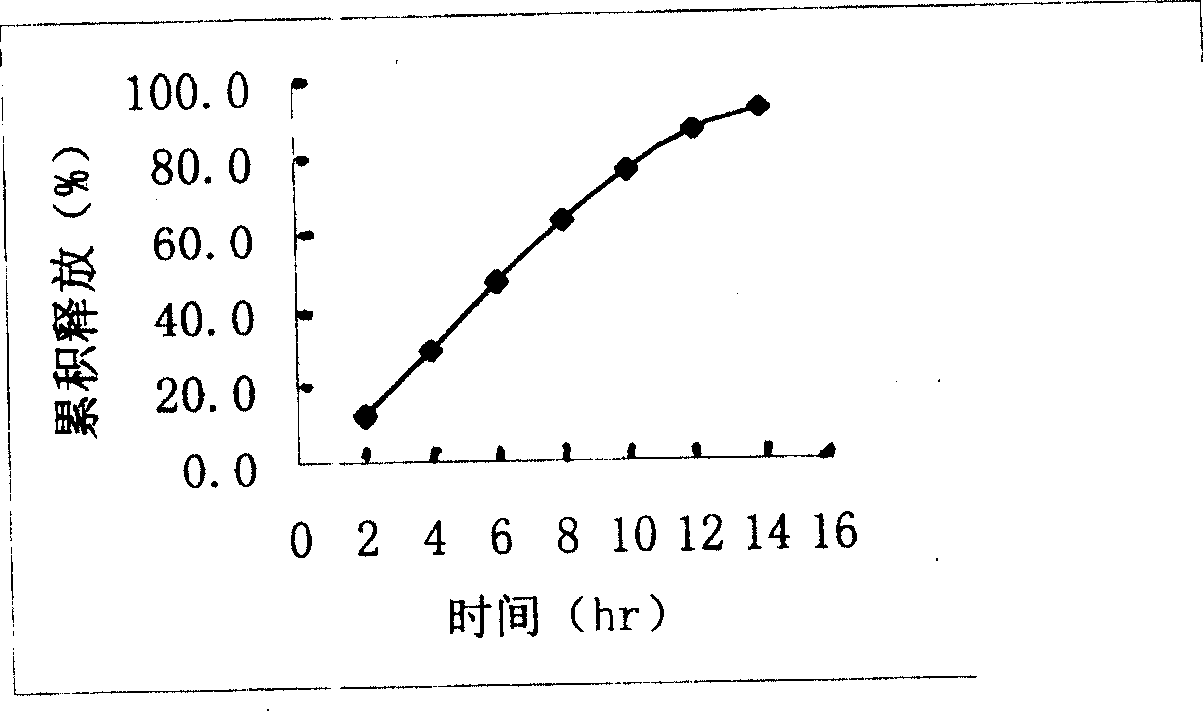
Image
Examples
Embodiment 1
[0018] The present embodiment 1 tablet that adopts the known method of pharmaceutical industry to make contains following composition by weight percentage:
[0019] Doxazosin Mesylate 2.7%
[0020] HPMCK4M (Methocel K15MCR) 45%
[0021] Lactose 51.6%
[0022] Magnesium Stearate 0.7%
[0023] HPMCK4M is a hydrophilic polymer, which is a skeleton material in this preparation, which swells with water or digestive juice to form a gel barrier to control the diffusion of doxazosin mesylate and achieve the purpose of sustained release.
[0024] Among them, HPMCK4M can be replaced by different dosages of HPMCK100LV, HPMCK15M or HPMCK100M, or used in combination to adjust the drug release curve.
Embodiment 2
[0026] The present embodiment 2 tablet that adopts the known method of pharmaceutical industry to make contains following composition by weight percentage:
[0027] Doxazosin Mesylate 3%
[0028] Carbopol 45%
[0029] Lactose 51%
[0030] Magnesium Stearate 1%
[0031] Hydrophilic polymer polycarboxyethylene (carbomer) is used as the skeleton material, which swells with water or digestive juice to form a gel barrier to control the diffusion of doxazosin mesylate and achieve the purpose of sustained release.
Embodiment 3
[0033] The present embodiment 3 tablet that adopts the known method of pharmaceutical industry to make contains following composition by weight percentage:
[0034] Doxazosin Mesylate 3%
[0035] Sodium Alginate 37%
[0036] Calcium Alginate 3%
[0037] Lactose 56%
[0038] Magnesium Stearate 1%
[0039] The insoluble salt and soluble salt of the hydrophilic polymer alginic acid are used as the skeleton material, which swells with water or digestive juice to form a cross-linked gel barrier to control the diffusion of doxazosin mesylate and achieve the purpose of sustained release.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com